Next: Answer one of the
Up: Chemistry 2720 Fall 1999
Previous: Answer all questions in
- Should we expect
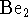 to be a stable molecule in the gas
phase? Explain briefly.
[3 marks]
to be a stable molecule in the gas
phase? Explain briefly.
[3 marks] - Element 118 has apparently recently been made at the Lawrence
Berkeley National Laboratory in California by fusing atoms of
krypton and lead. Based on quantum
mechanical principles, the chemical properties of this new
element should most closely resemble those of which existing
element(s)? Explain. [3 marks]
- Explain the meaning of the negative sign in the equation for the
energy of a hydrogenic atom. [3 marks]
- Briefly describe how the radial probability density corresponding
to a given atomic orbital can be
calculated. [3 marks]
Marc Roussel
Fri Feb 11 19:48:33 MST 2000