![]()
where T is in Kelvin. What is the entropy of solid aluminium oxide at the melting point?
- Relate the allowed de Broglie wavelengths to the radius of the ring. Show that a quantum number appears in this relation.
- Relate the kinetic energy to the de Broglie wavelength and thus to your quantum number.
- Calculate the angular momentum
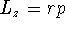 .
.
- According to modern quantum mechanics, the angular momentum
of a particle on a ring should be
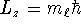 where
where 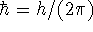 and
and 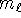 is an integer.
How does this relationship
compare with that derived in question 3c?
is an integer.
How does this relationship
compare with that derived in question 3c?
- The majority of compounds in thermodynamic tables have negative standard free energies of formation. For example, over 80% of the compounds listed in the Appendix of the course textbook have negative standard free energies of formation. Discuss briefly why this is not surprising.
- The standard free energies of formation of gaseous benzene
(
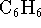 ) and
cyclohexane (
) and
cyclohexane ( 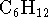 ) are, respectively,
129.66 and 31.76kJ/mol. One occasionally sees claims
in otherwise respectable textbooks that cyclohexane is
more stable than benzene because the free energy of
formation of cyclohexane is lower. Explain why this is
an inappropriate use of standard free energies of
formation.
) are, respectively,
129.66 and 31.76kJ/mol. One occasionally sees claims
in otherwise respectable textbooks that cyclohexane is
more stable than benzene because the free energy of
formation of cyclohexane is lower. Explain why this is
an inappropriate use of standard free energies of
formation. - For the reaction
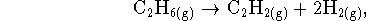
the entropy change is positive since excess gas molecules are produced. Since entropy is supposed to increase during spontaneous processes, this reaction should be spontaneous but it isn't. What's wrong?