Next: Answer any two questions
Up: Chemistry 2720 Fall 1998
Previous: Chemistry 2720 Fall 1998
Total value of this section: 80
- State the first and second laws of thermodynamics. [4 marks]
- Give an example of a natural phenomenon for which classical mechanics
has no explanation but which can be explained using quantum
mechanics.
[2 marks]
- Using
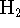 as an example, explain the difference between
bonding and antibonding molecular orbitals. [5 marks]
as an example, explain the difference between
bonding and antibonding molecular orbitals. [5 marks] - Do you expect
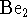 to be a stable molecule? Explain
briefly. [4 marks]
to be a stable molecule? Explain
briefly. [4 marks] - What is the ground state electronic configuration of a scandium
atom?
[4 marks]
- How much heat is released or absorbed when 50g of sodium
hydroxide is dissolved in water? [6 marks]
-
- One of the diamond industry's favorite advertising lines
is that ``diamonds are forever''. A reasonable
thermodynamic interpretation of this line is that
the reaction
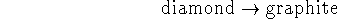
is not spontaneous under normal
conditions. Is this true?
[2 marks]
- In reactions which involve only solids or liquids, we
generally ignore any terms proportional to PV, such as
the work. Calculate the work
done when one mole of diamond is converted to one mole
of graphite at 298K under a constant pressure of
1atm. Based on your calculation, would it normally be
acceptable to neglect the work? The density of diamond
is
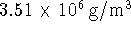 while the density of graphite is
while the density of graphite is
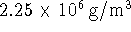 .
[8 marks]
.
[8 marks] - Calculate
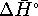 ,
, 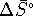 and
and 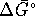 for the conversion of diamond
to graphite at
for the conversion of diamond
to graphite at 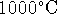 accurately.
[12 marks]
accurately.
[12 marks] - Calculate
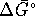 for the conversion of
diamond to graphite at
for the conversion of
diamond to graphite at 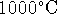 using the approximation that
using the approximation that
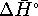 and
and 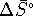 are
approximately constant and equal to their values at
298K. Compare your answer to that obtained in the
last part of this question. If you had not had enough
data to do a detailed calculation and had been forced to
use the approximate method, would the free energy change
obtained have been of any value? [3 marks]
are
approximately constant and equal to their values at
298K. Compare your answer to that obtained in the
last part of this question. If you had not had enough
data to do a detailed calculation and had been forced to
use the approximate method, would the free energy change
obtained have been of any value? [3 marks]
- The solubility of nitrogen in water at
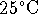 under
a pressure of pure nitrogen of 1atm is
under
a pressure of pure nitrogen of 1atm is
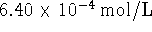 . Calculate the free energy
of formation of an aqueous nitrogen molecule. [5 marks]
. Calculate the free energy
of formation of an aqueous nitrogen molecule. [5 marks] - The solubility of nitrogen in water at
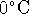 is
is 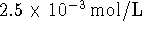 .
Calculate the enthalpy of formation of an aqueous
nitrogen molecule. [5 marks]
.
Calculate the enthalpy of formation of an aqueous
nitrogen molecule. [5 marks]
- Suppose that an electron in a box makes a transition from the
ground state to the first excited state while absorbing a photon
of wavelength 250nm. What is the length of the box? [10 marks]
- Some cells take in phosphate by reacting it with ADP to form ATP (and
water). This fulfills two cellular needs simultaneously, namely
phosphate uptake and ATP synthesis, but the process is not
spontaneous under realistic conditions. The driving force is
provided by the spontaneous flow of protons across the cell
membrane. The pH outside the cell is typically lower than the pH
inside by 1.5 units. Three protons flow
into the cell for every phosphate transported in.
If the concentration of phosphate outside the cell is
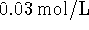 and
the concentration of ADP inside the cell is
and
the concentration of ADP inside the cell is 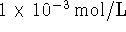 , what is the maximum concentration of
ATP which can be reached inside the cell at 298K due to this
process alone?
For the reaction
, what is the maximum concentration of
ATP which can be reached inside the cell at 298K due to this
process alone?
For the reaction
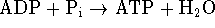 ,
,
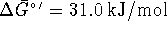 at 298K.
[10 marks]
at 298K.
[10 marks]
Next: Answer any two questions
Up: Chemistry 2720 Fall 1998
Previous: Chemistry 2720 Fall 1998
Marc Roussel
Fri Dec 18 21:22:47 MST 1998
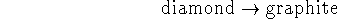
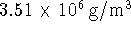 while the density of graphite is
while the density of graphite is
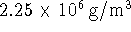 .
[8 marks]
.
[8 marks]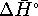 ,
, 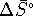 and
and 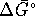 for the conversion of diamond
to graphite at
for the conversion of diamond
to graphite at 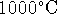 accurately.
[12 marks]
accurately.
[12 marks]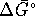 for the conversion of
diamond to graphite at
for the conversion of
diamond to graphite at 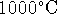 using the approximation that
using the approximation that
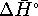 and
and 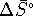 are
approximately constant and equal to their values at
298K. Compare your answer to that obtained in the
last part of this question. If you had not had enough
data to do a detailed calculation and had been forced to
use the approximate method, would the free energy change
obtained have been of any value? [3 marks]
are
approximately constant and equal to their values at
298K. Compare your answer to that obtained in the
last part of this question. If you had not had enough
data to do a detailed calculation and had been forced to
use the approximate method, would the free energy change
obtained have been of any value? [3 marks]
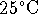 under
a pressure of pure nitrogen of 1atm is
under
a pressure of pure nitrogen of 1atm is
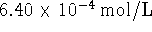 . Calculate the free energy
of formation of an aqueous nitrogen molecule. [5 marks]
. Calculate the free energy
of formation of an aqueous nitrogen molecule. [5 marks]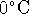 is
is 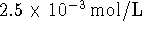 .
Calculate the enthalpy of formation of an aqueous
nitrogen molecule. [5 marks]
.
Calculate the enthalpy of formation of an aqueous
nitrogen molecule. [5 marks]