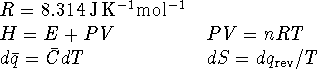Up: Back to the Chemistry 2720 test index
Chemistry 2720 Fall 1998 Test 1
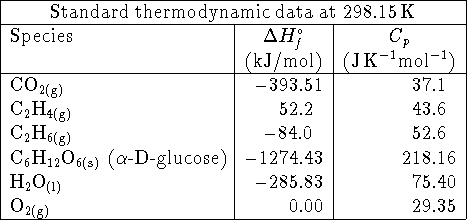
- Do you expect
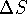 to be positive or negative for the
reaction
to be positive or negative for the
reaction
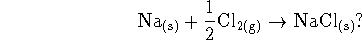
Explain briefly. [5 marks]
- Compare the enthalpy of combustion of glucose at
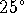 to
the enthalpy of combustion
at
to
the enthalpy of combustion
at 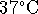 . In your opinion, is the difference
sufficiently small to ignore in
metabolic calculations? [10 marks]
. In your opinion, is the difference
sufficiently small to ignore in
metabolic calculations? [10 marks] - Catalytic hydrogenation converts unsaturated hydrocarbons
into saturated hydrocarbons by reaction with hydrogen gas.
How much heat is produced or absorbed per mole of ethene
(
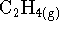 ) hydrogenated to ethane
(
) hydrogenated to ethane
( 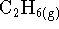 )
in a constant-volume reactor at 298K?
Indicate clearly whether heat is absorbed or produced.
[10 marks]
)
in a constant-volume reactor at 298K?
Indicate clearly whether heat is absorbed or produced.
[10 marks] - Calculate the entropy of water vapor at
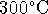 and
1atm.
The entropy of liquid water at
and
1atm.
The entropy of liquid water at 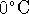 and 1atm
is
and 1atm
is 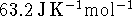 .
The constant-pressure specific heat capacity of liquid water
is
.
The constant-pressure specific heat capacity of liquid water
is 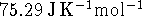 and the heat capacity of
water vapor
is
and the heat capacity of
water vapor
is 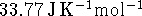 . The enthalpy of
vaporization of water at
. The enthalpy of
vaporization of water at 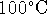 and 1atm is
40.66kJ/mol.
[10 marks]
and 1atm is
40.66kJ/mol.
[10 marks]
- Using your knowledge of thermodynamics, prove that steam at
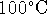 will condense in a room at
will condense in a room at
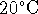 . Question 4
contains relevant data. [5 marks]
. Question 4
contains relevant data. [5 marks] - A condenser is a device which puts a vapor in thermal contact
with a substance (often water) which can absorb heat and
therefore cause the vapor to condense. Here is a simple diagram
of a condenser:
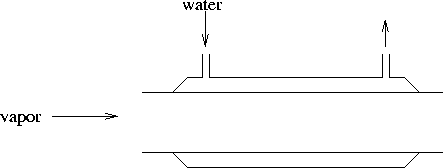
Water (from a tap) flows through the outer jacket. The vapor
condenses on the walls and then drips out of the device.
- In a small still, 1L of liquid ethanol is produced per hour.
The ethanol is not cooled appreciably below its boiling point
before entering the collection vessel.
How much heat is gained by the condenser water during an
hour?
Ethanol boils at
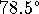 C and its enthalpy of
vaporization is 878.60J/g. The density of ethanol is
0.79g/mL. [5 marks]
C and its enthalpy of
vaporization is 878.60J/g. The density of ethanol is
0.79g/mL. [5 marks] - The tap water
enters the condenser at a temperature of
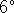 C
and
flows through the condenser at a rate of 20L/h.
The density of water is approximately constant and is
0.999g/mL.
For water,
C
and
flows through the condenser at a rate of 20L/h.
The density of water is approximately constant and is
0.999g/mL.
For water, 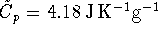 .
At what temperature is the water leaving the condenser?
[5 marks]
.
At what temperature is the water leaving the condenser?
[5 marks]
To convert degrees Celcius to Kelvin, add 273.15.

Up: Back to the Chemistry 2720 welcome page
Marc Roussel
Thu Oct 15 12:27:53 MDT 1998
![]()

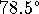 C and its enthalpy of
vaporization is 878.60J/g. The density of ethanol is
0.79g/mL. [5 marks]
C and its enthalpy of
vaporization is 878.60J/g. The density of ethanol is
0.79g/mL. [5 marks] C
and
flows through the condenser at a rate of 20L/h.
The density of water is approximately constant and is
0.999g/mL.
For water,
C
and
flows through the condenser at a rate of 20L/h.
The density of water is approximately constant and is
0.999g/mL.
For water, 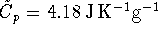 .
At what temperature is the water leaving the condenser?
[5 marks]
.
At what temperature is the water leaving the condenser?
[5 marks]