


Up: Back to the Chemistry 2720 assignments index
Chemistry 2720 Fall 2000 Assignment 1
Due: Tuesday, Sept. 19, 9:25a.m.
This assignment is intended to help you review material from
prerequisite courses useful in our study of thermodynamics.
If you find that you have weaknesses in certain
areas, you should address them during the first few weeks of classes.
You are expected to be able to recognize chemical names and transform
them to chemical formulas without the assistance of a periodic table, at
least for compounds of some of the more common elements. You should know
the formulas of several common complex ions. You should also be able to
give formulas for some of the common acids. Finally, you are
expected to know the formulas of a few common trivially named compounds.
Give chemical formulas for each of the following chemical species.
[1 mark each]
- 1.
- sodium hydrogen phosphate
- 2.
- xenon tetrafluoride
- 3.
- iron (III) oxide
- 4.
- cobalt (II) ion
- 5.
- hydrochloric acid
- 6.
- dinitrogen pentoxide
- 7.
- fluorine
- 8.
- calcium bromide
- 9.
- oxygen
- 10.
- ammonia
- 11.
- iodide ion
- 12.
- ammonium nitrate
- 13.
- nickel (II) sulfate hexahydrate
- 14.
- sodium chloride
- 15.
- potassium ion
- 16.
- methane
- 17.
- nitric acid
You should be able to balance chemical reactions, whether you are given
a complete set of reagents and products or just a verbal description of
the reaction.
Balance the following reactions. [3 marks each]
- 1.
- NaCl reacts with
SO2, water and oxygen.
The products are
Na2SO4 and HCl.
- 2.
- Combustion of ethanol (
CH3CH2OH)
You already know a certain amount of basic physical chemistry. Although
we will review most of this material as we need it, you are expected to
already be familiar with it. You may need to look up a few pieces of
data from the textbook to complete the questions in this section.
However, you should know the solubility rules well enough to predict
which ionic compounds will be soluble in water without reference to a
table.
- 1.
- Assuming ideal gas behavior, calculate the number of moles of
gas in a
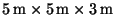 room at a pressure of 0.9bar and a temperature of
room at a pressure of 0.9bar and a temperature of
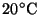 .
[5 marks]
.
[5 marks]
- 2.
- Calculate the amount of heat required to raise the temperature of
200g of water from 4 to
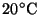 .
[5 marks]
.
[5 marks]
- 3.
- Calculate the amount of heat released when 400g of steam
condenses to liquid water at
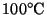 .
[4 marks]
.
[4 marks]
- 4.
- Given
| Substance |
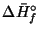 (kJ/mol)
(kJ/mol) |
|
BCl3(g) |
-403.8 |
|
BF3(g) |
-1136.0 |
|
Cl2(g) |
0 |
|
F2(g) |
0 |
calculate the change in enthalpy (
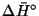 )
in the
reaction
)
in the
reaction
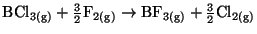 .
[4 marks]
.
[4 marks]
- 5.
- Relate the equilibrium constant for the reaction
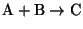 to the concentrations of A, B and C, assuming that the reaction
occurs in solution. [2 marks]
to the concentrations of A, B and C, assuming that the reaction
occurs in solution. [2 marks]
- 6.
- Determine the pH of a 0.01mol/L solution of acetic acid in
water. [5 marks]
- 7.
- Which of the following ionic compounds are likely to be completely soluble
in water? [1 mark each]
- (a)
-
PbSO4
- (b)
-
Na2SO4
- (c)
- FeS
- (d)
-
(NH4)2SO4
- (e)
-
Pb(NO3)2
- 8.
- Which of the following compounds are strong acids in aqueous
solution? [1 mark each]
- (a)
- HF
- (b)
-
H2SO4
- (c)
-
H3PO4
- (d)
- HCl
- (e)
-
HSO4-
Although the mathematical burden in this course is not particularly
heavy, you are expected to be able to work out elementary derivatives
and integrals.
- 1.
- Compute the derivative of the function
f(x) = 3x5+2x+1.
[2 marks]
- 2.
- Compute the following integrals.
- (a)
-
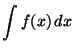 with f(x) as in question
1 [3 marks]
with f(x) as in question
1 [3 marks]
- (b)
-
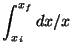 [3 marks]
[3 marks]
- (c)
-
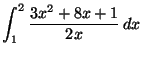 [5 marks]
[5 marks]



Up: Back to the Chemistry 2720 assignments index
Marc Roussel
2000-09-20
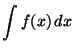 with f(x) as in question
1 [3 marks]
with f(x) as in question
1 [3 marks]
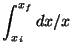 [3 marks]
[3 marks]
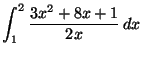 [5 marks]
[5 marks]