- For the mechanism

under what condition(s) would the equilibrium and steady-state approximations be valid?
- Obtain an approximate rate law using the steady-state approximation. Assuming the validity of the steady-state approximation, what would be the experimentally observed order of reaction?
- Suppose that
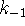 is so small that it can be neglected
and that
is so small that it can be neglected
and that 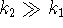 . Explain what approximations can
be used in this case and verify that
. Explain what approximations can
be used in this case and verify that
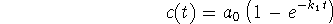
- Under the conditions described in question 1c,
if
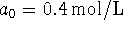 and
and 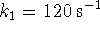 , how long would it take for the
concentration of product to rise to 0.2mol/L?
, how long would it take for the
concentration of product to rise to 0.2mol/L?

Obtain an approximate rate law for this mechanism valid if ![]() is large. Discuss the dependence of the rate of reaction on the
pressure of the inert gas X, paying particular attention to what
happens in the limit of large or small X. Also, from the point of
view of chemical realism, what problem(s) do you foresee with your
rate equation is X is very small?
is large. Discuss the dependence of the rate of reaction on the
pressure of the inert gas X, paying particular attention to what
happens in the limit of large or small X. Also, from the point of
view of chemical realism, what problem(s) do you foresee with your
rate equation is X is very small?
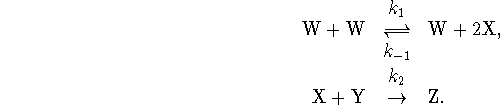
- What is the overall reaction? Identify the reactants, products and intermediates in the mechanism.
- Assuming that W is a normal molecule with fully satisfied valencies, what kind of chemical species is X?
- Suppose that the first step reaches equilibrium rapidly. Derive an approximate rate law valid in this case.
- Under what condition(s) could you apply the steady-state approximation? Derive an approximate rate law using this approximation.
![]()
where K is a ratio of rate constants. Then show, by
manipulating this equation, that a plot of a/v vs ![]() should be linear if a reaction obeys the Lindemann law. State
clearly the values of the intercept and slope.
should be linear if a reaction obeys the Lindemann law. State
clearly the values of the intercept and slope.
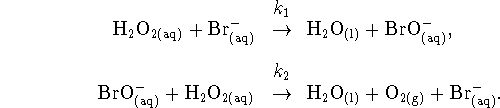
- What is the role of the bromide ions in this mechanism?
- The second step is much faster than the first. Apply an appropriate approximation to obtain the rate law for this mechanism.
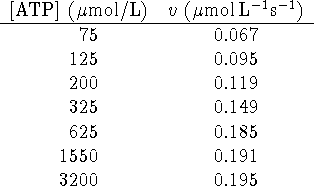
Calculate ![]() and
and ![]() for this enzyme/substrate
system. Comment on the fit of the data to the Michaelis-Menten
rate law.
for this enzyme/substrate
system. Comment on the fit of the data to the Michaelis-Menten
rate law.
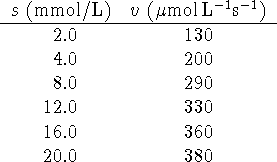
Given that the concentration of enzyme is 2.0g/L and that its
molar mass is approximately 50kg/mol, calculate ![]() and
and
![]() .
The sum
.
The sum ![]() appearing in the expression for
appearing in the expression for ![]() derived from the steady-state approximation (
derived from the steady-state approximation ( ![]() ) is of course
no smaller than
) is of course
no smaller than ![]() . From this observation, compute a
lower bound for the value of
. From this observation, compute a
lower bound for the value of ![]() .
.