- Why can salt be used to melt ice? [3 marks]
- Ethanol is used as a deicing agent for airplane wings and in other applications where a solid deicing agent would be inconvenient. However, it is never used on roads. Can you suggest a reason why? Hint: One of your senses can be used to alert you to the presence of ethanol. [2 marks]
- What is the pH of a solution made by dissolving
10g of NaOH (molar mass 39.997g/mol) in 200mL of
water at
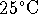 ? [5 marks]
? [5 marks] - 100mL of 6.0mol/L HCl is added to the solution of question 4a. What is the pH after this addition? [5 marks]
- The
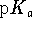 's of the three protons of phosphoric
acid (
's of the three protons of phosphoric
acid ( 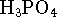 )
are 2.1, 7.2 and 12.3, all at
)
are 2.1, 7.2 and 12.3, all at 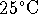 .
Do you think that acid phosphates
could be used to make an effective pH 4
buffer? Why or why not? [2 marks]
.
Do you think that acid phosphates
could be used to make an effective pH 4
buffer? Why or why not? [2 marks] - Suppose that you want to make a pH 12 buffer. You have bottles of phosphoric acid (molar mass 97.9951g/mol), sodium dihydrogen phosphate (119.9769g/mol), sodium hydrogen phosphate (141.9588g/mol) and sodium phosphate (163.9407g/mol). Which of these chemicals will you need to make the buffer? [2 marks]
- You want to make 10L of a pH 12 buffer with a total concentration of phosphates of 0.50mol/L. What masses of which of the above chemicals do you need to dissolve in the 10L of water to make the buffer? [8 marks]
![]()
at ![]() is
is ![]() .
.
- Describe the equilibrium state of a mixture of lead and
lead azide left in an open container at
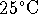 under normal atmospheric conditions.
The partial pressure of nitrogen in the atmosphere is 0.79atm.
[2 marks]
under normal atmospheric conditions.
The partial pressure of nitrogen in the atmosphere is 0.79atm.
[2 marks] - 1kg of lead azide comes to equilibrium
in a 3L sealed box at
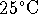 . The box initially
contains 1atm of pure nitrogen. What is the
equilibrium nitrogen pressure in the box? [6 marks]
. The box initially
contains 1atm of pure nitrogen. What is the
equilibrium nitrogen pressure in the box? [6 marks]