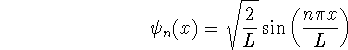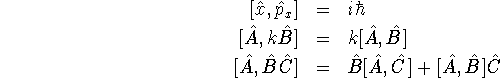Up: Back to the Chemistry 3730 test
index
Chemistry 3730 Spring 1997 Test 1
Please note the useful information at the end of this paper.
Aids permitted: calculator, Maple.
Your solutions should explain exactly what computations need to be
performed, even if Maple is used to help with the algebra.
- State whether each of the following sets of hydrogenic states
is possible or impossible. Give the quantum numbers for
possible states. For the incorrect states, explain which rule
is being violated. [6 marks]
(a) 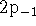 (b)
(b) 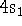 (c)
(c) 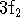
- Calculate the average kinetic energy for a particle in a
one-dimensional box in the ground and first excited states.
[10 marks]
-
- Explain what is meant by ``compatible observables''.
[2 marks]
- In one dimension, are position and kinetic energy compatible
observables?
[8 marks]
-
- Calculate the approximate energies of the ground state and of the
first excited state of a particle in a
one-dimensional box of length L in which the particle
experiences a potential energy
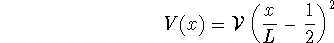
as a function of position. [10 marks]
- State the condition(s) under which you expect your
answers to question 4a to be accurate.
[2 marks]
- Compare the energy levels of a particle in a plain
box to your answers to question 4a.
In which case is the difference greatest? Why?
[2 marks]
Hint: Sketch V(x).
- Calculate the probability that an electron in a hydrogen
atom is between 0 and
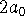 distance units away from the nucleus
in the 1s and 2s states. Then calculate the probability that
the electron is between
distance units away from the nucleus
in the 1s and 2s states. Then calculate the probability that
the electron is between 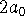 and
and 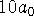 from the nucleus
in the same two
states. Round your answers to 4 decimal places.
The 1s and 2s wavefunctions are
from the nucleus
in the same two
states. Round your answers to 4 decimal places.
The 1s and 2s wavefunctions are
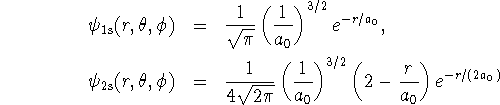
[10 marks]
Maple hint: 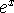 is
is exp(x) in Maple.
The number  is Pi in Maple.
is Pi in Maple.
For a particle in a one-dimensional box of length L, the wavefunctions are
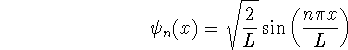
and the corresponding energies are

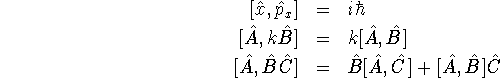
In spherical polar coordinates, the volume element is
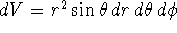 . Conventionally,
. Conventionally, 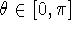 and
and 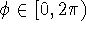 .
.



Up: Back to the Chemistry 3730 test
index
Marc Roussel
Tue Feb 4 14:44:12 MST 1997
![]() (b)
(b) ![]() (c)
(c) ![]()
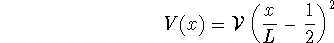
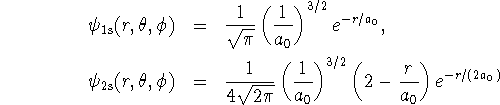
![]() is
is