- How many valence electrons would lithium have in this alternate Universe? [2 marks]
- Name the next element in lithium's group in this alternate Universe. [2 marks]
![]()
Determine the values of A, B and k. [10 marks]
Useful fact:
![]()
- What kind of spectrometer would you need to measure the bond stiffness k for a heteronuclear diatomic molecule? [2 marks]
- The fundamental vibrational transition of
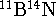 occurs
at
occurs
at
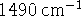 . Calculate k for this molecule
assuming harmonic-oscillator behavior.
The isotopic masses of
. Calculate k for this molecule
assuming harmonic-oscillator behavior.
The isotopic masses of 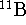 and
and 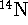 are
are 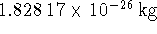 and
and
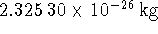 , respectively.
[10 marks]
, respectively.
[10 marks]
An extension of HMO theory for heteroatoms gives the ![]() orbital energies of methanimine (
orbital energies of methanimine ( ![]() )
as
-1.2808 and 0.7808 while those
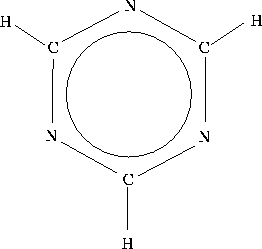
of the aromatic molecule 1,3,5-triazine (Fig. 1)
are
-2.2656, -1.2808 (2), 0.7808 (2), 1.7656.
All orbital
energies are given relative to the carbon Coulomb integral
(
)
as
-1.2808 and 0.7808 while those
of the aromatic molecule 1,3,5-triazine (Fig. 1)
are
-2.2656, -1.2808 (2), 0.7808 (2), 1.7656.
All orbital
energies are given relative to the carbon Coulomb integral
( ![]() ) in units of the bond (``resonance'')
integral
) in units of the bond (``resonance'')
integral ![]() .
.
One empirical aromaticity scale suggests that benzene and 1,3,5-triazine are roughly equally aromatic, i.e. that they are subject to a similar degree of resonance stabilization. Do the Hückel calculations agree? [10 marks]