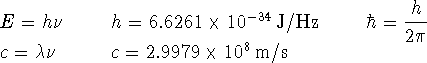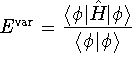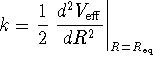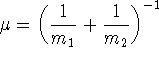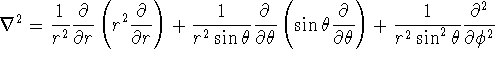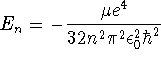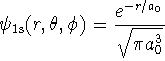![]()
in molecular problems. Suppose that we wanted to approximate the ground-state wavefunction of a hydrogen atom by the above Gaussian function. What would the percent error in the energy be? [10 marks]
Maple hints: The exponential function ![]() is exp(u)
in Maple and
is exp(u)
in Maple and ![]() is Pi.
Maple will need to know that c>0. Use subs() to
substitute your best value of c into your variational energy,
then use simplify() to get an initial simplification.
You will need to complete the simplification process by hand.
is Pi.
Maple will need to know that c>0. Use subs() to
substitute your best value of c into your variational energy,
then use simplify() to get an initial simplification.
You will need to complete the simplification process by hand.
![]()
occurs at 217nm.
Calculate the value of the resonance integral ![]() from
this information. Note that
from
this information. Note that ![]() is negative.
is negative.
![]()
where R is the internuclear distance and D, ![]() and
and
![]() are parameters specific to a particular molecule.
are parameters specific to a particular molecule.
- Show that D is the asymptote of the potential, i.e. the
limit of V as
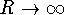 .
.
Maple hint: If you use Maple for this problem, Maple will need to know that
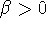 .
. - Show that
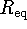 is the equilibrium bond length.
is the equilibrium bond length. - Calculate k as a function of the parameters D and
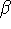 .
.
Maple hints: Use subs() to substitute the equilibrium bond length into the expression and simplify() to clean up the answer.
- Give an expression for the dissociation energy.
Maple hint: ![]() is Pi in Maple.
is Pi in Maple.