


Up: Back to the Chemistry 3730 test
index
Chemistry 3730
Fall 1995 Final Examination
Aggregate value of this examination: 40 marks.
Answer all questions in this section. Aggregate value of questions in
this section: 24.
- In intense laser fields, two-photon processes sometimes occur.
In a two-photon process, two photons are simultaneously absorbed
by a molecule (hence the name).
- State
an essential condition for the simultaneous
absorption of two photons by a molecule.
- Derive
the selection rule for the change in the
electronic angular momentum quantum number
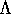 during a two-photon process.
during a two-photon process.
- For a two-level system
in a radiation field, the wavefunction
can be written as a time-dependent superposition of the pure
states
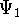 and
and 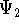 :
:
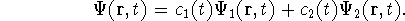
The coefficients 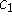 and
and 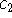 evolve according to
evolve according to
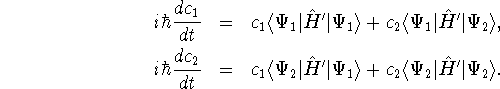
In these equations, 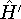 is the perturbation induced by
the radiation field.
Explain what these equations tell us about absorption and
emission of radiation by matter.
is the perturbation induced by
the radiation field.
Explain what these equations tell us about absorption and
emission of radiation by matter.
-
- State the Bloch theorem.
- Explain why the
Krönig-Penney model leads to bands of allowed
electronic energies.
- Distinguish
electrical insulators and conductors on the
basis of the band theory.
- Describe in detail a spectroscopic
method for measuring a molecular parameter. You may choose
any parameter in any type of molecule but you must
specify the type of spectroscopic information required and relate
this as precisely as possible (using equations if possible)
to the parameter you have chosen. Make sure to accurately define the
parameter whose measurement you will describe.
- The fluorescence lifetime
of a certain chemical group
is measured and found to be 8ns. A quencher is added
at a concentration of 0.2mol/L
and the fluorescence lifetime decreases to 3ns.
What is the rate constant
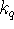 for the bimolecular
quenching process?
for the bimolecular
quenching process? - A chemical reaction is
carried out between the fluorescent molecule
and quencher of question 5a whose
product contains both the fluorescent (donor) and
quenching (acceptor) groups.
A solution of this chemically modified molecule is
prepared.
If the fluorescence lifetime of the donor is 1ns,
what is the rate constant
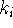 for intramolecular quenching?
(In class, we called this process excitation transfer.)
for intramolecular quenching?
(In class, we called this process excitation transfer.) - Set
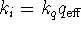 and calculate
and calculate
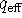 , the
effective intramolecular quencher concentration.
What information does this number convey?
, the
effective intramolecular quencher concentration.
What information does this number convey?
Answer one of the following two questions, for six marks.
-
- Give
the Hamiltonian and boundary conditions for the
particle-in-a-box problem.
- Apply
the variational method to the particle-in-a-box
problem using a sine function as your trial wavefunction.
Do you get all of the particle-in-a-box solutions by
this method? Discuss.
- Using the trial wavefunction
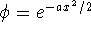 ,
compute the approximate
ground state energy of the one-dimensional
quartic oscillator with
Hamiltonian
,
compute the approximate
ground state energy of the one-dimensional
quartic oscillator with
Hamiltonian
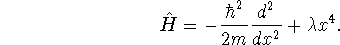
The following integral will be useful:
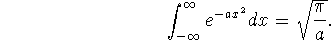
Answer one of the following two questions, for ten marks.
- Write down
the Born-Oppenheimer electronic Hamiltonian
for LiH. If you use summation symbols in your
Hamiltonian (a practice which I recommend), make sure to
write down the summation limits explicitly.
- Write down
the ground state electronic wavefunction of LiH as a
Slater determinant of molecular orbitals.
- Write down
a Slater determinant for an excited state
of LiH. How does it differ from the ground state Slater
determinant?
- Explain
how you would go about determining approximate
formulas for the LiH molecular orbitals at a particular
internuclear separation R.
- Explain why I specified ``at a
particular internuclear separation'' in the previous
question in relation to Born-Oppenheimer theory.
- Describe in detail
a method for obtaining an accurate ground-state
energy for the helium atom. Show all relevant equations and
discuss any difficulties which you anticipate in their solution.
Also briefly describe how excited state energies might be
obtained.
For a Hermitian operator 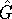 ,
,
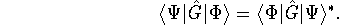



Up: Back to the Chemistry 3730 test
index
Marc Roussel
Fri Jan 3 16:23:26 MST 1997
 during a two-photon process.
during a two-photon process.
![]()
![]() and
and ![]() evolve according to
evolve according to
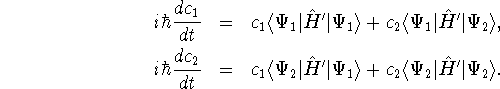
![]() is the perturbation induced by
the radiation field.
Explain what these equations tell us about absorption and
emission of radiation by matter.
is the perturbation induced by
the radiation field.
Explain what these equations tell us about absorption and
emission of radiation by matter.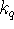 for the bimolecular
quenching process?
for the bimolecular
quenching process? 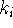 for intramolecular quenching?
(In class, we called this process excitation transfer.)
for intramolecular quenching?
(In class, we called this process excitation transfer.)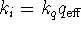 and calculate
and calculate
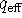 , the
effective intramolecular quencher concentration.
What information does this number convey?
, the
effective intramolecular quencher concentration.
What information does this number convey?