Up: Back to the Chemistry 3730 test index
Chemistry 3730 Fall 2000 Quiz 4
Name:
Note: You should start the computations for questions 1-3
while you work on question 4.
- 1.
- Perform a geometry optimization on trans-2-butene
at the
3-21G level. Report the energy,
bond lengths, and the C-C=C bond angle.
[7 marks]
Suggestion: To avoid disasters, save your structure to disk
after the computation.
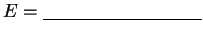 kcal/mol kcal/mol | C-C: Å |
|
CH3 C-H: Å | C=C: Å |
| =C-H: Å | C-C=C angle:  |
- 2.
- Repeat the calculation for cis-2-butene:
Are there significant quantitative differences (other than the
obvious change in stereochemistry) between the two structures?
Discuss briefly.
[10 marks]
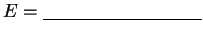 kcal/mol kcal/mol | C-C: Å |
|
CH3 C-H: Å | C=C: Å |
| =C-H: Å | C-C=C angle:  |
- 3.
- Compute
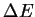 for the reaction
for the reaction
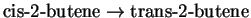 .
For a simple gas-phase isomerization such as this one,
.
For a simple gas-phase isomerization such as this one,
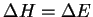 .
The experimental value of
.
The experimental value of 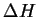 for this
reaction is
for this
reaction is
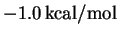 .
How does your value compare? Is the
agreement (or lack thereof) expected?
[5 marks]
.
How does your value compare? Is the
agreement (or lack thereof) expected?
[5 marks]
- 4.
- The rotational absorption spectrum of 1H35Cl
consists of a series of roughly equally spaced lines separated
by
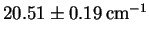 .
What is the bond length?
[10 marks]
.
What is the bond length?
[10 marks]
Notes: Don't bother propagating the uncertainty unless you're curious
about the uncertainty in the bond length.
The rotational selection rule is
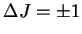 .
The
rotational energy of a diatomic molecule is given by
.
The
rotational energy of a diatomic molecule is given by
The isotopic masses of 1H and of
35Cl are, respectively, 1.0078250321 and
34.96885271amu. The reduced mass is defined by
Finally,
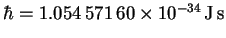 ,
the atomic mass unit corresponds to
,
the atomic mass unit corresponds to
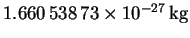 and
and
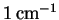 is
is
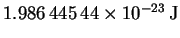 .
.



Up: Back to the Chemistry 3730 test index
Marc Roussel
2000-11-20
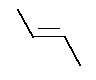
![]() .
The
rotational energy of a diatomic molecule is given by
.
The
rotational energy of a diatomic molecule is given by