- (a)
- Briefly describe the photoelectron spectroscopy
experiment. In particular, explain what needs to be measured
to obtain the final result. [6 marks]
Notes: While it may help to write down a formula or two to guide your discussion, very little mathematical detail is expected. In particular, you need not give any detail about how any relevant quantities are measured.
- (b)
- Predict the photoelectron spectrum of N2 using Koopmans's theorem. [9 marks]
- (a)
- Using an argument based on LCAO-MO theory, predict the
bond order in the molecular ion
HHe+.
[3 marks]
Hint: If it helps, treat this case by analogy to an isoelectronic homonuclear diatomic molecule.
- (b)
- Compute the bond length in HHe+. [5 marks]
- (c)
- Predict the frequencies of the first several lines of
the rotational absorption spectrum of
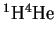 given that the
isotopic mass of
given that the
isotopic mass of
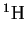 is 1.0078250321amu
while the mass of
is 1.0078250321amu
while the mass of
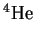 is
4.0026032497amu.
[7 marks]
is
4.0026032497amu.
[7 marks]