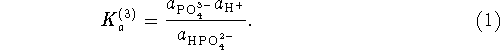![]()
To save typing, I will call these two compounds G6P and F6P from here on. The reaction is spontaneous if
![]()
For this reaction,
![]() . To make
. To make ![]() negative, Q must be sufficiently small, i.e. there must be
enough reactant relative to the product concentration.
You can solve this problem by trial and error with your
calculator. I find that a ratio of reactant to product of 2:1
(i.e.
negative, Q must be sufficiently small, i.e. there must be
enough reactant relative to the product concentration.
You can solve this problem by trial and error with your
calculator. I find that a ratio of reactant to product of 2:1
(i.e. ![]() ) is adequate to get a negative
) is adequate to get a negative
![]() so, for example, we could have a concentration
of F6P of 1mM and a concentration of G6P of 2mM.
so, for example, we could have a concentration
of F6P of 1mM and a concentration of G6P of 2mM.
![]()
Therefore
![]()
Calculating ![]() is easy after that:
is easy after that:
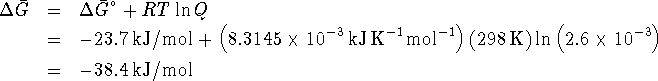
The maximum work available is therefore 38.4kJ/mol of ethanol produced.
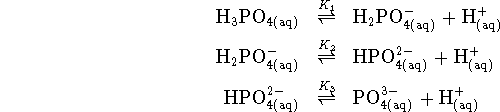
Using the free energies of formation of the phosphates, we find

We now find the ![]() 's of the three steps using the formula
's of the three steps using the formula ![]() :
:

It is convenient (but not essential) to convert these acid
dissociation constants into ![]() 's:
's:

At the ![]() , an acid and its conjugate base are
present in equal amounts. At pH's far above
, an acid and its conjugate base are
present in equal amounts. At pH's far above ![]() ,
the acid form is present in only very small amounts. It follows
that at pH 11.5, phosphoric acid exists almost exclusively as
the phosphate and hydrogen phosphate anions. Thus, to a very
good approximation, we need only consider the last equilibrium.
For this equilibrium,
,
the acid form is present in only very small amounts. It follows
that at pH 11.5, phosphoric acid exists almost exclusively as
the phosphate and hydrogen phosphate anions. Thus, to a very
good approximation, we need only consider the last equilibrium.
For this equilibrium,
Furthermore
(the total concentration of phosphates is 0.01mol/L and we
know that the other phosphate species are present only in tiny
amounts) and ![]() . We now have two equations in two unknowns. Solving
eq 1 for , we get
. We now have two equations in two unknowns. Solving
eq 1 for , we get
Substituting this relation into eq 2, we get a simple equation in one unknown with solution
![]()
We substitute this result back into eq 3:
![]()
Once we have found the concentrations of the phosphate and hydrogen phosphate anions, we can work backwards through the other equilibria. For the second equilibrium,
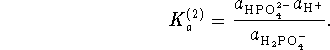
Since we know everything that goes into this formula except for , we can solve for this quantity:
![]()
Similarly,
![]()
Since the standard concentration is 1mol/L, the concentrations of phosphates in the buffer are
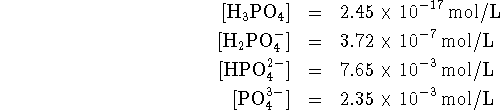
![]()
The amount of ![]() in solution is a combination of
protons released by water and by HCl:
in solution is a combination of
protons released by water and by HCl:
This relation and the ionization constant equation
provide us with the two equations we need to find our two
unknowns. ( ![]() , which you can look up in any first-year
textbook, has the value
, which you can look up in any first-year
textbook, has the value ![]() at 298K.)
If we solve eq 4 for
at 298K.)
If we solve eq 4 for ![]() and substitute this into eq 5, we get
and substitute this into eq 5, we get
![]()
We solve this equation using the quadratic equation:
![]()
The pH is therefore 6.96.
![]()
For this particular mixture at ![]() (973K), we have
(973K), we have
![]()
It is impossible to tell whether these two substances will mix at this temperature. We know that the free energy of mixing must be larger than this small negative number, but we don't know how much larger. It could be slightly negative (in which case the two substances will mix) or it could be positive (in which case they won't).