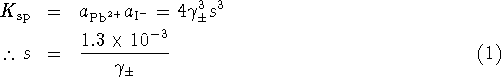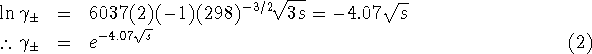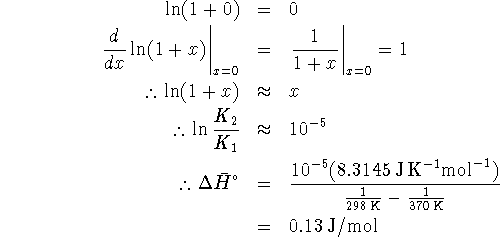![]()
at 298K. The equilibrium constant at this temperature is
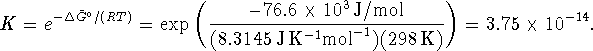
We want the equilibrium constant at 323K. To calculate this,
we need ![]() :
:
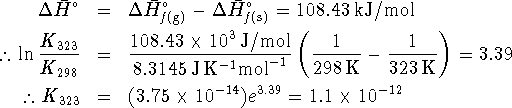
The vapour pressure of sodium at ![]() is
therefore
is
therefore ![]() .
.
![]()
Thus
![]()
or ![]() . The number of moles of
the protein is therefore
. The number of moles of
the protein is therefore
![]()
and the molar mass is
![]()
Molar masses of this magnitude are usually expressed as 193kg/mol.
![]()
Note that ![]() is unaffected because the
standard free energies of formation of the glucoses cancel out.
The maximum ratio of glucose in to glucose out is obtained when
all of the available free energy is begin used up by transport,
i.e. when
is unaffected because the
standard free energies of formation of the glucoses cancel out.
The maximum ratio of glucose in to glucose out is obtained when
all of the available free energy is begin used up by transport,
i.e. when ![]() . This implies that
. This implies that
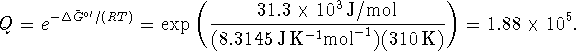
In terms of the activities,
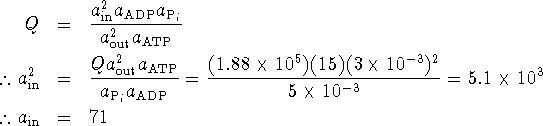
The glucose concentration in the cell can be as high as 71mol/L.
![]()
Let s be the molar solubility. Then
To use the Debye-Hückel equation, we need to express the ionic strength in terms of s.
![]()
The Debye-Hückel equation becomes
Use ![]() as an initial guess (i.e. assume ideal
behaviour) to kick off chain iteration of equations 1
and 2.
as an initial guess (i.e. assume ideal
behaviour) to kick off chain iteration of equations 1
and 2.
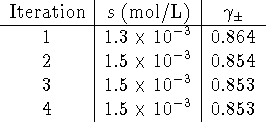
The solubility of lead (II) iodide is ![]() .
.