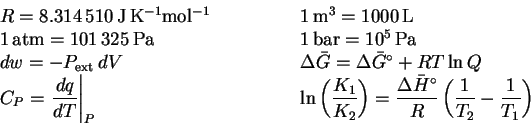Up: Back to the Chemistry 2720 test index
Chemistry 2720 Fall 2000 Test 1
Formulas and data can be found at the end of this paper.
Write your answers only in the booklet provided. Use extra pages at the
back of the booklet for scratch work.
- 1.
- As the temperature
of a kernel of popcorn increases, two things happen:
- The starch and protein which make up most of the
kernel cook.
- The water heats up, eventually exceeding its boiling point.
When the water in the kernel vaporizes, it causes a tremendous
increase in pressure, which literally causes the kernel to
explode. The cooked starch and protein dehydrate rapidly, making
the popped corn crispy and fluffy.1
- (a)
- A typical kernel of popcorn has a moisture content of
approximately 14% (by mass).
How much heat is required
to completely vaporize the moisture in a 15g serving
of popcorn starting from
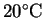 ?
Assume that the water vaporizes at its normal boiling point
and that the process occurs at constant pressure.2
[8 marks]
?
Assume that the water vaporizes at its normal boiling point
and that the process occurs at constant pressure.2
[8 marks]
- (b)
- Suppose that a kernel of popcorn can hold a pressure of
1.5bar before breaking.3 What temperature would the
water inside reach before boiling?
Neglect the fact that the water may contain some
solutes.
[10 marks]
- (c)
- Unlike most reactions involving solids, popcorn expands
spectacularly when it explodes.
Suppose that you
pop 20mL (initial volume)
of a variety that expands by a factor of 40 when
popped.4
How much expansion work does the popcorn do if the
atmospheric pressure is 0.9bar (typical for
Lethbridge)? [6 marks]
- 2.
- The overall reaction in an alkaline battery is
- (a)
- Calculate the maximum electrical work which an alkaline
battery can perform per kilogram of zinc (molar mass
65.38g/mol) at
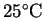 .
[7 marks]
.
[7 marks]
- (b)
- If an alkaline battery initially contains 10g of zinc
and 15g of manganese (IV) oxide (molar mass
86.936g/mol),
what is the maximum total electrical
work which can be produced at
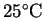 ?
[8 marks]
?
[8 marks]
- 3.
- The solubility of thallium (IV) fluoride (
TlF4) in
water at
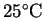 is
is
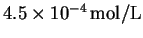 .
.
- (a)
- What is the standard free energy change for the dissociation
reaction?
[8 marks]
- (b)
- Suppose that you wanted to use the above result to
determine the standard free energy of formation of thallium (IV)
fluoride. How would you proceed? What additional data
would you need? [5 marks]
To convert degrees Celsius to Kelvin, add 273.15.
The specific heat capacity of liquid water is
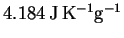 and its enthalpy of vaporization at
and its enthalpy of vaporization at
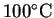 is 2257J/g or
40.66kJ/mol.
is 2257J/g or
40.66kJ/mol.
For liquids and solids, a=X. For solutes,
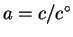 ,
where
,
where
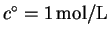 in the usual definition of the
standard state. For gases,
in the usual definition of the
standard state. For gases,
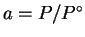 ,
where
,
where
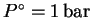 .
.
in the chemists' standard state at a standard temperature of
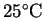 and a standard pressure of 1bar.
and a standard pressure of 1bar.
| Species |
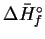 |
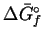 |
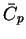 |
| |
(kJ/mol) |
(kJ/mol) |
(JK-1mol-1) |
|
MnO2(s) |
-520.0 |
-465.2 |
54.1 |
|
Mn2O3(s) |
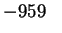 |
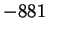 |
107.7 |
|
ZnO(s) |
-350.5 |
-320.5 |
40.3 |
Footnotes
- ... fluffy.1
- H. McGee, On
Food and Cooking, Collier: New York, 1984, p 242. Incidentally,
this book makes for excellent pleasure reading.
- ... pressure.2
- Most
of the water probably vaporizes after the kernel
breaks so that this is a sensible approximation.
- ... breaking.3
- This is a pure guess.
Relatively little is known about the chemistry and
physics of popcorn.
- ... popped.4
- This
expansion factor is at the low end of typical values.
Source: www.popcorn.org. (I'm not making this
up.)



Up: Back to the Chemistry 2720 test index
Marc Roussel
2000-10-19
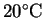 ?
Assume that the water vaporizes at its normal boiling point
and that the process occurs at constant pressure.2
[8 marks]
?
Assume that the water vaporizes at its normal boiling point
and that the process occurs at constant pressure.2
[8 marks]
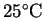 .
[7 marks]
.
[7 marks]
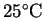 ?
[8 marks]
?
[8 marks]