Therefore
![\begin{eqnarray*}\Delta\bar{H}^\circ & = & \Delta\bar{H}^\circ_{f(\mathrm{H_2CO_...
...93.51 + (-285.830)]\,\mathrm{kJ/mol} =
-20.36\,\mathrm{kJ/mol}
\end{eqnarray*}](img2.gif)
The number of moles of CO2 is
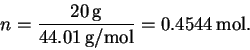
The total enthalpy change is therefore
- (a)
- We worked out the equation for the work done during
the reversible, isothermal expansion of an ideal gas in
class:
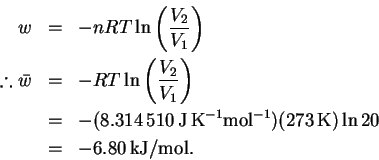
Note that this is an expansion so the work is negative (work done by the system). - (b)
- The work per mole is calculated by
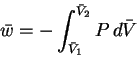
To do the integral, we must first write P as a function of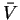 :
:
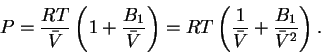
Thus
![\begin{eqnarray*}\bar{w} & = & -RT\int_{\bar{V}_1}^{\bar{V}_2}d\bar{V}\left(\fra...
...mathrm{m^3/mol}}\right)\right]\\
& = & -6.79\,\mathrm{kJ/mol}
\end{eqnarray*}](img9.gif)
The correction hardly has any effect on the calculated value of the molar work.
- (a)
- The block is so hot that it will vaporize all the water.
The final temperature will be greater than
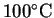 .
.
- (b)
- The block is hot enough to vaporize only some of the
water. The final temperature will be exactly
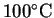 .
.
- (c)
- The block is not hot enough to raise the temperature of
the water to
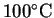 .
All the water
remains in the liquid state.
.
All the water
remains in the liquid state.
We have
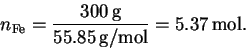
The heat released as the iron block cools from 150 to
Far more heat is required to raise the temperature of the water to
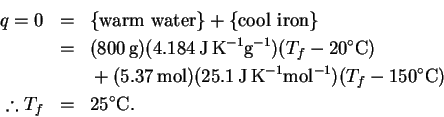
will either produce or absorb heat, leading to a change in temperature of the water. The molar enthalpy of reaction is
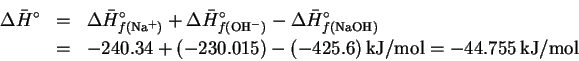
The number of moles of sodium hydroxide is
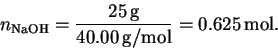
Thus
The reaction is exothermic, so the temperature will increase. We will need to do a computation with the heat capacity of water to determine the temperature rise. Molar and mass-specific heat capacities are available, so we need to convert the volume to one or the other set of units. Using the density of water at
The heat balance equation is
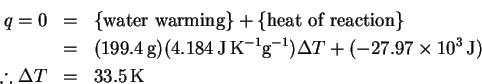
This is not so large a temperature change as to make this process very dangerous (the final temperature will be well below the burn temperature for human skin), but it will clearly be necessary to stir the solution as we go to avoid creating hot spots due to slow diffusion of the heat away from the site of dissolution.