The following funding agencies are acknowledged for generous support of our research (PI grants and student scholarships):

Research in the Hayes group involves the synthesis of high-energy and reactive inorganic molecules for application in new chemical transformations and catalysis. Four unique projects directly address this goal.

One research avenue being pursued is to tackle the challenge of preparing new materials that are both biodegradable and biocompatible. A particularly attractive alternative to conventional polyolefin materials is polylactide, PLA, which is generated by the ring-opening polymerization of lactide, the cyclic ester dimer of lactic acid. PLA is desirable because of its processability and useful macroscopic properties. The synthetic precursors to PLA are found in inexpensive renewable resources, such as beets and corn. Furthermore, the resultant polymers are biodegradable and have found applications in such areas as bulk packaging materials and the agricultural industry. It has also garnered interest from the medical community for its use in adsorbable sutures, as matrices for the slow release of pharmaceuticals, and polymer scaffolds for tissue engineering.
Despite these benefits, the industrial process for PLA synthesis involves a melt polymerization protocol in the presence of a metal containing catalyst. This method offers very little control over the molecular weights and stereochemistry of the final product; also, the metal catalyst remains embedded within the polymer. Our strategy focuses upon development of discrete homogeneous lactide polymerization catalysts, specifically, alkaline earth metal alkyl, alkoxide and amido species.[1] Homogeneous systems are highly advantageous because they allow rational fine tuning of the steric and electronic environment of the metal centre.
Initial forays into this avenue of investigation involved the preparation of zinc complexes of a neutral phosphinimine ligand featuring a dibenzofuran scaffold, which yielded complexes catalytically active in the polymerization of l-lactide at 100 °C.[2]

More recently, we have demonstrated that highly active cationic magnesium complexes of a neutral bis(phosphinimine) ligand were suitable for the preparation of poly(ε-caprolactone) at ambient temperature in several minutes.[3].

The preparation of analogous cationic organozinc complexes has recently been achieved[4].

Further studies will provide the opportunity to produce materials of defined molecular weights containing specific macroscopic properties, while ultimately serving as a platform for the discovery of new materials. As a better understanding of the catalyst systems unfold, the steric environment of the ancillary ligand set will be fine tuned for stereospecific polymerization by introduction of chiral functionalities. Stereochemistry of the polymer is particularly important because it determines its mechanical and physical properties, and also, its rate of chemical and biological degradation.
In summary, this project explores the virtually uncharted organometallic chemistry of non-Cp supported alkaline earth metals with the possibility of contributing significantly to the fundamental understanding of the production of environmentally friendly materials.
| [1] | Wheaton, C. A.; Hayes, P. G.; Ireland, B. J. "Complexes of Mg, Ca and Zn as homogeneous catalysts for lactide polymerization" Dalton Trans. 2009, 4832-4846. (FEATURED ON COVER OF ISSUE) |
| [2] | Wheaton, C. A.; Ireland, B. J.; Hayes, P. G. "Activated Zinc Complexes Supported by a Neutral Phosphinimine-Containing Ligand: Synthesis and Efficacy for the Polymerization of Lactide" Organometallics 2009, 28, 1282-1285-6361. |
| [3] | Ireland, B. J.; Wheaton, C. A.; Hayes, P. G. "Cationic Organomagnesium Complexes as Highly Active Initiators for the Ring Opening Polymerization of ε-Caprolactone" Organometallics 2010, ASAP. |
| [4] | Wheaton, C. A.; Hayes, P. G. "Cationic organozinc complexes of a bis(phosphinimine) pincer ligand: synthesis, structural and polymerization studies" Dalton Trans. 2010, In Press. |
Another project in the Hayes research group involves the preparation of novel tridentate pincer ligands to support low-valent and metal main-group multiple bonding within lanthanide and group 3 metals. Although several examples of complexes with group 3 metals and the lanthanides in oxidation states other than the ubiquitous +3 have been reported, there are few, if any, unambiguous examples of isolated M(I) or M(II) species (where M = Sc, Y, La, Lu, Ho, Er, Pr) prepared by conventional synthetic techniques.
Using a novel bis(phosphinimine)carbazole ligand, we have prepared a thermally sensitive dialkyl lutetium(III) complex.[1]

At temperatures above 0 °C, this compound undergoes two sequential intramolecular ortho-metallation processes.

It is expected that, with further studies in this area, complexation of our class of ligands (which will be designed to impart thermal stability in the product) will generate new platforms for obtaining rare bonding motifs which will lead to the discovery of unique reaction behaviour and catalytic activity. For example, low-valent complexes may allow for access to ligands such as CO or olefins, which generally bind very weakly, if at all, to these metals due to a lack of backbonding from the metal centre. Such complexes are likely to be of fundamental interest, but also useful from an applications perspective; olefin-bound species may serve as reasonable models for intermediates in olefin polymerization catalysis; thus, providing a unique glimpse at otherwise fleeting structures.
| [1] | Johnson, K. R. D.; Hayes, P. G. "Synthesis and Reactivity of of Dialkyl Lutetium Complexes Supported by a Novel Bis(phosphinimine)carbazole Pincer Ligand" Organometallics 2009, 28, 6352-6361. |
Another thrust of the Hayes group research program is the development of catalytic systems for converting hydrocarbons into useful organic compounds. Such derivatization is especially desirable for smaller hydrocarbons, such as methane, which contribute significantly to the production of greenhouse gases. The metal-mediated coupling of an alkane with an alkene is an example of such a useful transformation.

This research utilizes phosphido ancillary ligands to synthesize reactive early (groups 3-5) and late (groups 8-10) transition metal complexes for the activation of chemically inert small molecules. A specific emphasis is placed upon the selective functionalization of hydrocarbons produced as undesirable side products in the petrochemical industry.

The isolation of complexes with this ligand motif should provide insight into fundamental questions regarding bonding and structure, as well as topics with more immediately tangible benefits, such as rational improvements of current catalytic systems and the development of entirely novel reactions. The short term objectives of this project are to generate metal complexes with unusual structures and oxidation states, with an eye to the ultimate goal of achieving catalytic functionalization of hydrocarbons and other small molecules.
A final project in the Hayes group is the study of inorganic polymers using solid-state NMR spectroscopy.[1] This is a collaboration between the Hayes group and Dr. Paul Hazendonk. The work focuses specifically on a class of polymers known as poly(phosphazenes), which are hybrid inorganic-organic materials of great interest due to their potential applications in novel electronics, biomaterials and biodegradable plastics.
We have demonstrated that by using 31P magic-angle spinning (MAS) solid-state (SS) NMR spectroscopy, the complicated thermal ring-opening polymerization of hexachlorocyclotriphosphazene can be quantitatively probed to give information about reaction progress, chain propagation and cross-linking of the resultant polymer.[2]

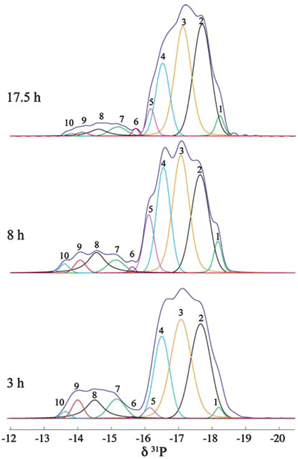
Deconvolution analysis of the SS 31P NMR spectra of a reaction mixture aliquots.
In another contribution, it was shown that the detailed morphology of another polymer, poly[bis(trifluoroethoxy)phosphazene, could be probed using MAS SS NMR spectroscopy.[3] For instance, the degree of crystalline versus amorphous domains in a given sample can be determined with a specialized pulse sequence. Furthermore, it was demonstrated that SS NMR is a viable technique for assigning specific crystalline domains (e.g. α-, β- or γ-phases) and may prove to be a valuable complementary technique to X-ray diffraction methods.
| [1] | Borisov, A. S.; Hazendonk, P.; Hayes, P. G. "Solid-State Nuclear Magnetic Resonance Spectroscopy: A Review of Modern Techniques and Applications for Inorganic Polymers" J. Inorg. Organomet. Polym. Mater. 2010, Submitted. |
| [2] | Borisov, A. S.; Hazendonk, P.; Hayes, P. G. "31P MAS NMR Spectroscopy of Hexachlorocyclotriphosphazene at Different Stages During Thermal Ring-Opening Polymerization" J. Inorg. Organomet. Polym. Mater. 2010, ASAP. |
| [3] | Borisov, A. S.; Hazendonk, P.; Hayes, P. G. "A Morphological Study of Poly[bis(trifluoroethoxy)phosphazene] Using High Resolution Solid-State 13C NMR Spectroscopy" J. Inorg. Organomet. Polym. Mater. 2008, 18, 163-174. |