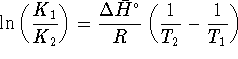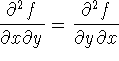Up: Back to the old Chemistry 2720 index
Chemistry 2720 Spring 1998 Test 2
Aids allowed: calculator.
Write your answers only in the booklets provided.
Answer exactly four questions of the following six questions.
All questions are equally weighted. Any additional questions answered
will not be marked.
-
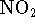 is a radical which can dimerize to
is a radical which can dimerize to
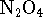 .
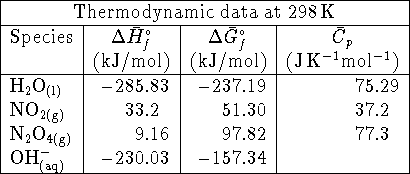
What is the equilibrium ratio of
.
What is the equilibrium ratio of
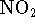 to
to 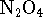 at 298K
if rigid container is initially filled with
0.5atm of
at 298K
if rigid container is initially filled with
0.5atm of 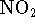 ? Treat both compounds as ideal
gases.
? Treat both compounds as ideal
gases. - Suppose that the Earth had a much lighter atmosphere, generating
a surface pressure of 0.2atm. Could liquid water exist on
Earth at this pressure? The normal boiling point of water is
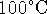 and the enthalpy of vaporization of water
at this temperature is 40.66kJ/mol.
and the enthalpy of vaporization of water
at this temperature is 40.66kJ/mol.
Hint: Treat the enthalpy of vaporization as a constant.
-
- Relate S to a derivative of A.
- Derive a Maxwell's relation for
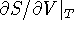 ,
i.e. use the interchangeability of the order of
differentiation to obtain this derivative.
,
i.e. use the interchangeability of the order of
differentiation to obtain this derivative. - What is
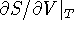 for an ideal gas?
for an ideal gas?
- A
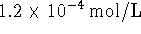 solution of NaOH is prepared. What is the pH
of this solution at
solution of NaOH is prepared. What is the pH
of this solution at 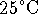 and at
and at 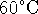 ?
? - Briefly explain each of the following concepts:
- coupled reactions
- Le Chatelier's principle
Try to focus on the underlying thermodynamic principles.
Illustrate your discussion with examples, if possible. - Suppose that a cell maintains its internal environment at a
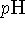 of
7.5 when the external medium has a
of
7.5 when the external medium has a 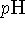 of 5.
This is achieved by the use of a proton pump powered by the
hydrolysis of ATP:
of 5.
This is achieved by the use of a proton pump powered by the
hydrolysis of ATP:
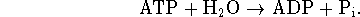
One ATP molecule is hydrolyzed for every two
protons transported across the membrane.
The standard free energy change for the hydrolysis at 298K in
the biochemists' standard state is -31.0kJ/mol. The total
concentration of phosphates within the cell is 2.8mmol/L.
What is the minimum ratio of ATP to ADP in this cell?
To convert degrees Celsius to Kelvin, add 273.
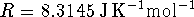
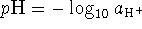
For an ideal gas, PV=nRT.
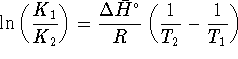
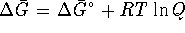
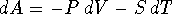
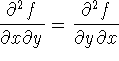

Up: Back to the old Chemistry 2720 index
Marc Roussel
Mon Mar 23 10:28:04 MST 1998
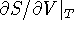 ,
i.e. use the interchangeability of the order of
differentiation to obtain this derivative.
,
i.e. use the interchangeability of the order of
differentiation to obtain this derivative.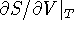 for an ideal gas?
for an ideal gas?
![]()