- The Carnot formula does not consider friction and other sources of loss which, unfortunately, cannot be avoided in real machines. However, the possibility that a very low friction mechanism has been found which makes the efficiency nearly equal to the theoretical maximum cannot entirely be excluded.
- A Carnot engine is the most efficient type of engine possible. Unless the inventor's engine is just a Carnot engine (in which case it may not be sufficiently original to merit patent protection), it must be less efficient than a Carnot engine. This point must be determined by inspecting the patent application.
- The engine is claimed to produce power at a reasonable rate. The Carnot formula applies strictly only to reversible engines. However, a reversible engine works infinitely slowly so it would produce no power. Since any irreversible engine (producing work at a reasonable rate) must be less efficient than a reversible engine, the inventor's claim is invalid.
- Place the sample in a pot of boiling water. Leave it
there for 10 minutes. We know that the water is at its
boiling point and, after leaving adequate time for thermal
equilibration, the brass should also reach this
temperature. (By the way, the boiling temperature of
water varies with altitude so it's not necessarily
exactly
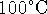 .)
.) - Meanwhile, carefully weigh a sample of room-temperature water
into an insulated vessel.
Place a thermometer in the water and measure its
temperature.
Make sure that the temperature is
stable, neither rising nor falling with time. Call this
temperature
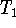 .
. - Remove the brass from the boiling water and place it quickly into the insulated vessel with the water.
- Record how the temperature changes over time. Stop the
experiment when the temperature of the water stabilizes.
Call this temperature
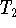 .
. - Dry and weigh the sample of brass.
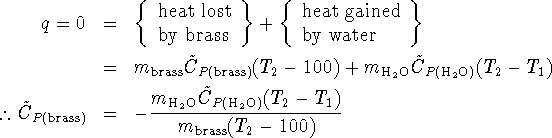
![]()
Using the conversion factor of 4.184J/cal, we find that
![]()
Warm beer provides 196kcal of energy per pint but 19kcal must be used up warming it to body temperature so the net energy gain is only 177kcal.
- At constant pressure,
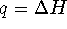 .
Since the heat per mole of HgS is required, the easiest
way to do this problem is to rewrite the reaction so
that it involves one stoichiometric equivalent of this
solid:
.
Since the heat per mole of HgS is required, the easiest
way to do this problem is to rewrite the reaction so
that it involves one stoichiometric equivalent of this
solid:
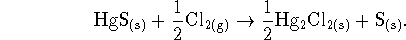
Then
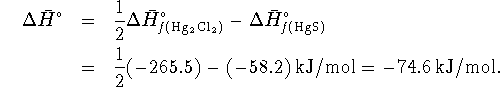
Since
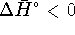 , this represents
74.6kJ/mol of heat produced by the reaction.
, this represents
74.6kJ/mol of heat produced by the reaction. - At constant volume,
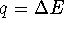 . Since E=H-PV,
. Since E=H-PV,
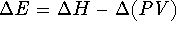 . If we only consider
the gases and treat them as ideal, we get
. If we only consider
the gases and treat them as ideal, we get
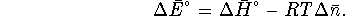
Again, we use the version of the chemical equation in which HgS has a stoichiometric coefficient of 1 since we want to know how much heat is produced per mole of this compound. In this case,
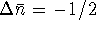 . Accordingly,
. Accordingly,
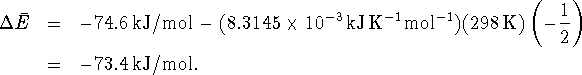
Thus, at constant volume, 73.4kJ of heat is produced for each mole of HgS consumed by the reaction.
- I will continue to use the form of the chemical equation
given on this page. It doesn't matter how you write the
equation provided it's properly balanced and you're
consistent.
First, calculate
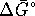 , from tables:
, from tables:
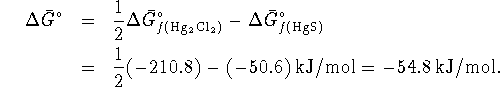
Now compute the free energy change when
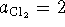 . Note that the solids all have an
activity of 1:
. Note that the solids all have an
activity of 1:
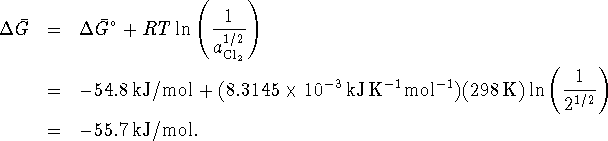
Since
 , the reaction is spontaneous.
, the reaction is spontaneous.
- We are interested in the combustion of methanol
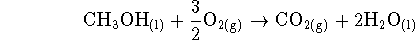
at
 . To get the enthalpy change at
this temperature, we need to use a path for which we
have all the requisite information. Consider the
sequence
. To get the enthalpy change at
this temperature, we need to use a path for which we
have all the requisite information. Consider the
sequence
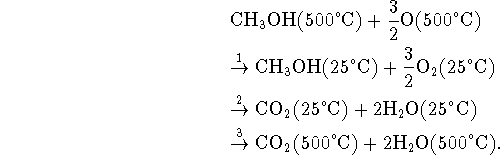
Step 2 is just the combustion at
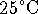 for which we have data.
In step 1,
for which we have data.
In step 1,
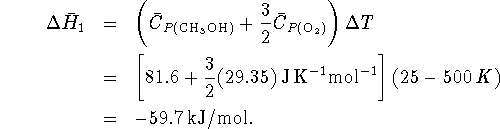
In step 3,
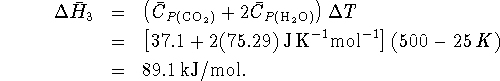
Thus for the overall reaction at the target temperature,
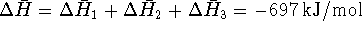 . Per liter, this is
. Per liter, this is
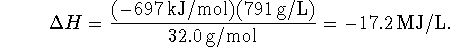
- The internal combustion engine produces work from heat
(it burns methanol) so it's a heat engine. Its
efficiency is
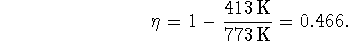
Note that we have to convert the temperatures to Kelvin before using this formula.
Burning methanol at a rate of 2L/h, it produces heat at a rate of 34.4MJ/h (computed using the answer to the previous part of this question). Thus
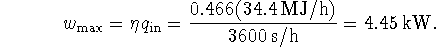
- If instead of burning the methanol it is oxidized
isothermally, free energy sets the maximum work
available and thus the maximum power which can be
developed. First, calculate
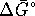 :
:
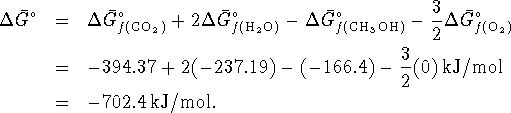
The free energy change under atmospheric conditions is then
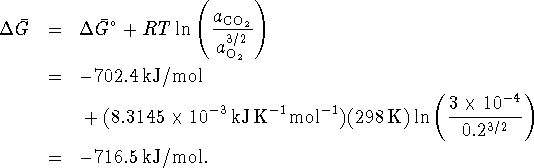
Now use the density and molar mass to convert this to
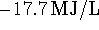 . Finally, use the methanol
consumption rate to calculate the power developed:
9.83kW. Note that this is more than double the power
developed by the heat engine.
. Finally, use the methanol
consumption rate to calculate the power developed:
9.83kW. Note that this is more than double the power
developed by the heat engine.