
Up: Back to the old Chemistry 2720 index
Chemistry 2720 Spring 1998 Test 1
Aids allowed: calculator.
Write your answers only in the booklets provided.
Answer all questions.
The questions in this test have an aggregate value of 50 marks.
Depending on your preferred style of questions, you may find it
worthwhile to solve the problems out of order.
- Following in Albert
Einstein's footsteps, you are working as a clerk at the patent
office. An inventor claims to have created a heat engine which
produces power at a rate of 5kW at maximum thermodynamic
efficiency, i.e. with an efficiency given exactly by
the Carnot formula.
Because the patent office will not award
patents to machines that cannot possibly function as claimed,
you are asked to decide whether the inventor's claim lies within
the realm of the possible.
What is your expert opinion?
Support your decision using your
knowledge of thermodynamics. [5 marks]
- After quitting the patent office (the pay is lousy),
you become a chemistry teacher (the pay is still lousy, but at
least you feel that you're playing a socially useful role).
You want your students to measure a heat capacity to help them
develop some intuition about this quantity and its relationship
to heat and temperature. You decide to have them measure the
heat capacity of brass, a conveniently unreactive solid, and one
whose heat capacity varies somewhat from one sample to another
so that they can't just look up the value in a table. Give a
brief procedure which your students should follow. Clearly
indicate what they should measure and describe the data handling
which will lead them from the raw data to a specific heat
capacity. [8 marks]
Note and hint: Brass is an alloy (a mixture of metals).
Because its composition varies somewhat from one sample to
another, it doesn't make sense to talk about moles of brass.
The heat capacity should therefore be expressed in
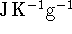 or in some other similar units.
or in some other similar units.
- A pint of beer delivers approximately 196kcal of metabolizable energy.
This figure assumes that the beer is served warm.
Cold beer must
also be warmed to body temperature (
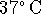 ) and
this requires an expenditure of metabolic energy.
Calculate the metabolizable
energy (in kcal) available from a pint of
beer served at
) and
this requires an expenditure of metabolic energy.
Calculate the metabolizable
energy (in kcal) available from a pint of
beer served at 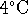 . Treat the beer as if its
thermal properties were identical to those of water. The
density of beer is typically just slightly higher than that of
water (about 1g/mL) and a pint is 568mL.
[6 marks]
. Treat the beer as if its
thermal properties were identical to those of water. The
density of beer is typically just slightly higher than that of
water (about 1g/mL) and a pint is 568mL.
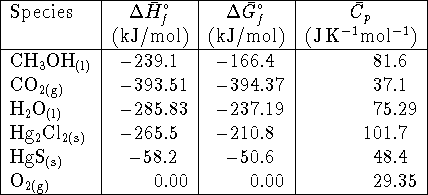
[6 marks] - All parts of this question refer to the reaction
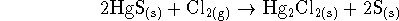
at the standard temperature (298K).
- Calculate the heat produced or absorbed
per mole of HgS consumed if the reaction occurs at
constant pressure. Indicate clearly whether
you think the reaction absorbs or produces heat.
[3 marks]
- Calculate the heat produced or absorbed per mole of
HgS consumed if the reaction occurs at constant
volume. [5 marks]
Hint: Assume that any gases involved in the reaction
behave ideally.
- Is the reaction spontaneous if the pressure of
chlorine gas is a constant 2atm? [5 marks]
-
- The enthalpy of combustion of liquid methanol (producing
liquid water) at
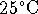 is
is 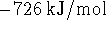 . Calculate the enthalpy of
combustion at
. Calculate the enthalpy of
combustion at 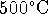 in kJ/L. The molar
mass of methanol is 32.0g/mol and the density is
791g/L. [8 marks]
in kJ/L. The molar
mass of methanol is 32.0g/mol and the density is
791g/L. [8 marks] - An internal combustion engine burns methanol at
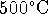 and produces exhaust gases with a temperature of
and produces exhaust gases with a temperature of
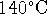 . If it consumes methanol at a
rate of 2L/h, how much power can it produce?
Assume that this engine is as efficient as permitted by
the laws of thermodynamics. [5 marks]
. If it consumes methanol at a
rate of 2L/h, how much power can it produce?
Assume that this engine is as efficient as permitted by
the laws of thermodynamics. [5 marks] - If a device oxidizes methanol isothermally
at 298K under atmospheric conditions (
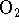 pressure of 0.2atm and
pressure of 0.2atm and 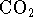 pressure of
pressure of 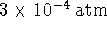 )
at the rate
of 2L/h, how much power would it produce? Again,
assume that the device operates without loss and at
maximum thermodynamic efficiency. Assume also that
power is produced in a form other than PV work.
[5 marks]
)
at the rate
of 2L/h, how much power would it produce? Again,
assume that the device operates without loss and at
maximum thermodynamic efficiency. Assume also that
power is produced in a form other than PV work.
[5 marks]
Hint: Oxidizing methanol would have the same overall
reaction as
combustion.
To convert degrees Celsius to Kelvin, add 273.
Power is measured in Watts (1W = 1J/s).
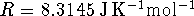
The constant pressure specific heat capacity of liquid water is
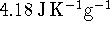 .
.
1cal = 4.184J
For an ideal gas, PV=nRT.
H = E + PV
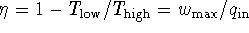
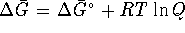

Up: Back to the old Chemistry 2720 index
Marc Roussel
Fri Feb 13 10:31:06 MST 1998
![]() or in some other similar units.
or in some other similar units.![]()
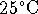 is
is 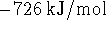 . Calculate the enthalpy of
combustion at
. Calculate the enthalpy of
combustion at 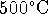 in kJ/L. The molar
mass of methanol is 32.0g/mol and the density is
791g/L. [8 marks]
in kJ/L. The molar
mass of methanol is 32.0g/mol and the density is
791g/L. [8 marks]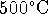 and produces exhaust gases with a temperature of
and produces exhaust gases with a temperature of
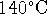 . If it consumes methanol at a
rate of 2L/h, how much power can it produce?
Assume that this engine is as efficient as permitted by
the laws of thermodynamics. [5 marks]
. If it consumes methanol at a
rate of 2L/h, how much power can it produce?
Assume that this engine is as efficient as permitted by
the laws of thermodynamics. [5 marks]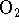 pressure of 0.2atm and
pressure of 0.2atm and 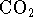 pressure of
pressure of 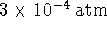 )
at the rate
of 2L/h, how much power would it produce? Again,
assume that the device operates without loss and at
maximum thermodynamic efficiency. Assume also that
power is produced in a form other than PV work.
[5 marks]
)
at the rate
of 2L/h, how much power would it produce? Again,
assume that the device operates without loss and at
maximum thermodynamic efficiency. Assume also that
power is produced in a form other than PV work.
[5 marks]
