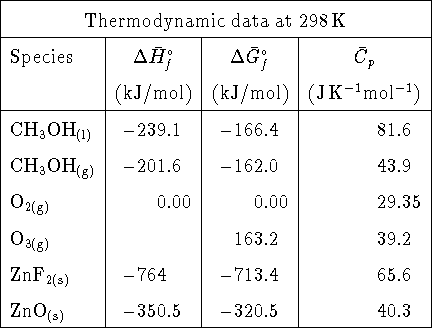
Notes: All sodium and nitrate salts are extremely soluble. You will need to use Debye-Hückel theory for the sodium nitrate/silver iodide mixture.
![]()
Complete the derivation of the osmotic pressure equation from this point. [10 marks]
Value of this section: 10 marks
Notes: All sodium and nitrate salts are extremely soluble. You will need to use Debye-Hückel theory for the sodium nitrate/silver iodide mixture.
![]()
Complete the derivation of the osmotic pressure equation from this point. [10 marks]
To convert degrees Celsius to Kelvin, add 273.
Liquid water has a specific heat capacity of ![]() .
Steam has a specific heat capacity of
.
Steam has a specific heat capacity of ![]() .
The enthalpy of vaporization of water at its normal boiling point
(
.
The enthalpy of vaporization of water at its normal boiling point
( ![]() ) is 2257J/g.
) is 2257J/g.
![]()
1W = 1J/s
![]()
dE = dw + dq
![]()
![]()
H = E+PV
G = H-TS
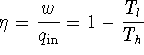
![]()
![]()
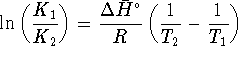
![]()
![]() with
with ![]()
![]()
![]()