Next: Thermodynamic data at 298K
Up: Back to the old Chemistry 2720 index
Chemistry 2720 assignment 1
Due: Friday, January 23, noon.
Most of the data you need is right in the assignment, but some of it
is not and can be found in any textbook.
- The ideal gas law is only valid for most gases at very low
temperatures. Real gases are more accurately described by the
van der Waals equation:
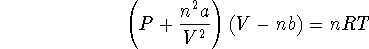
where a and b are constants specific to a particular gas,
R is the ideal gas constant, P is the pressure, V the
volume and n the number of moles.
- Derive an equation for the work done during a reversible,
isothermal expansion of a van der Waals gas.
[8 marks]
- For nitrogen,
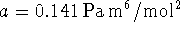 and
and
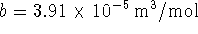 . Calculate
the work done when 1kmol of nitrogen is expanded
reversibly and
isothermally at a temperature of 300K from 1 to
. Calculate
the work done when 1kmol of nitrogen is expanded
reversibly and
isothermally at a temperature of 300K from 1 to
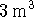 . [2 marks]
. [2 marks]
- The constant pressure heat capacity of the metal indium is given
by the equation
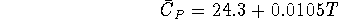
where T is in Kelvin and 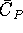 is in
is in
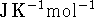 .
.
- Calculate the amount of heat required to raise the
temperature of a 12g piece of indium from 50 to
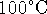 . [6 marks]
. [6 marks] - How large an error would result from using a (constant)
average value of
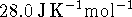 ?
[4 marks]
?
[4 marks]
- 5.2g of potassium bromide is dissolved in 100g of water
initially at
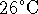 in an insulated container held at constant
pressure. Assume that the heat capacity of the solution is
approximately equal to the heat capacity of the water alone
(usually a reasonable approximation) and calculate the final
temperature. [10 marks]
in an insulated container held at constant
pressure. Assume that the heat capacity of the solution is
approximately equal to the heat capacity of the water alone
(usually a reasonable approximation) and calculate the final
temperature. [10 marks] - Lead melts at 600.6K. The enthalpy of melting is 4.774kJ/mol.
Suppose that a company has an
electrical furnace (a device that converts electrical work
completely into heat) that melts 100kg of lead per hour.
The lead enters the furnace at a
temperature of
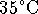 .
Assuming that no heat is lost, how
much electrical power is required to operate the furnace?
[10 marks]
.
Assuming that no heat is lost, how
much electrical power is required to operate the furnace?
[10 marks]
Hints:
- Power is measured in Watts. A Watt is a Joule
per second.
- Lead has a constant-pressure heat capacity of
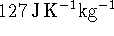 .
.
Next: Thermodynamic data at 298K
Up: Back to the old Chemistry 2720 index
Marc Roussel
Tue Jan 20 09:43:35 MST 1998
![]()
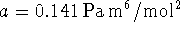 and
and
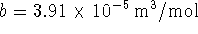 . Calculate
the work done when 1kmol of nitrogen is expanded
reversibly and
isothermally at a temperature of 300K from 1 to
. Calculate
the work done when 1kmol of nitrogen is expanded
reversibly and
isothermally at a temperature of 300K from 1 to
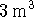 . [2 marks]
. [2 marks]
![]()
![]() is in
is in
![]() .
.
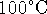 . [6 marks]
. [6 marks]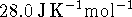 ?
[4 marks]
?
[4 marks]
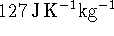 .
.