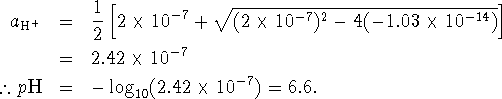Since HI is a strong acid, it completely dissociates in water. However, because the concentration is so low, we must consider the water autoionization equilibrium. The reaction is
![]()
For this reaction
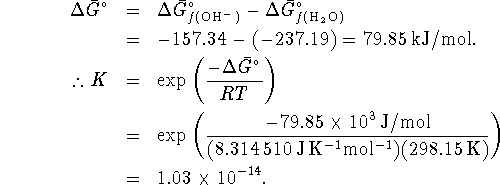
The equilibrium constant is related to the activities by
![]()
The charge balance equation for this solution is
![]()
with ![]() . Combining
these last two equations, we have
. Combining
these last two equations, we have
![]()
which we can rearrange to the quadratic equation
![]()
The positive root of this equation is