From the enthalpy of vaporization at the boiling point, we can compute
the enthalpy of vaporization at ![]() .
The following diagram traces out the path to be used for this
computation:
.
The following diagram traces out the path to be used for this
computation:
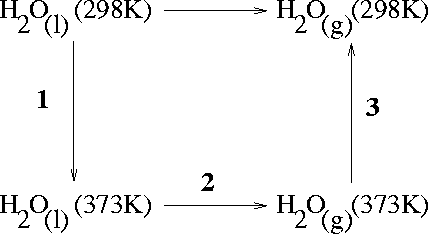
We compute the enthalpy changes in steps 1, 2 and 3 and add them to get the enthalpy of vaporization at the standard temperature:
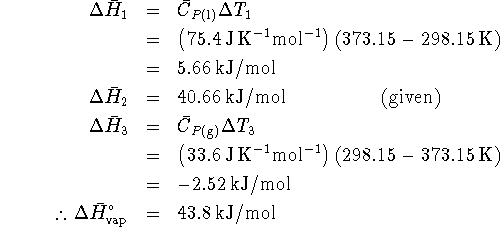
However, ![]() .
Therefore
.
Therefore
![]()
The accepted value (in case you're curious) is
![]() . The above calculation, which doesn't take
into account the variation of heat capacity with temperature, gives a
very good result.
. The above calculation, which doesn't take
into account the variation of heat capacity with temperature, gives a
very good result.