![]()
The enthalpy of reaction at ![]() is
is
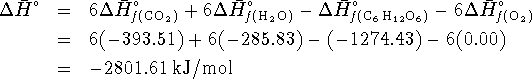
To get the enthalpy of reaction at ![]() ,
consider the following thermodynamic cycle:
,
consider the following thermodynamic cycle:
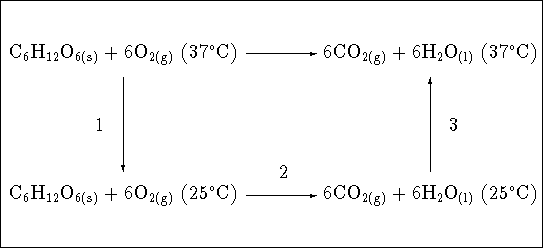
![]() , the enthalpy change at
the standard temperature (
, the enthalpy change at
the standard temperature ( ![]() ). We need
to calculate
). We need
to calculate ![]() and
and ![]() :
:
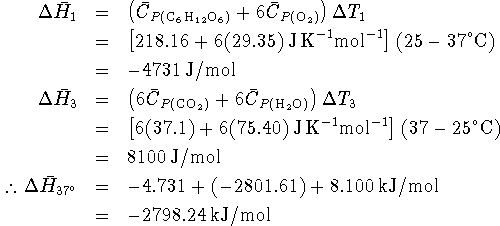
The difference between the two is about 3kJ/mol out of 2800 (about 0.1%). Metabolic studies are rarely this accurate so the difference is probably not significant.
![]()
For this reaction,
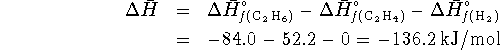
We want the heat at constant volume, i.e. ![]() .
.
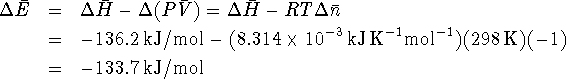
The reaction is exothermic, i.e. heat is produced.
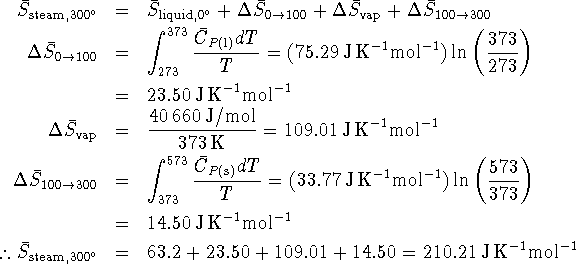
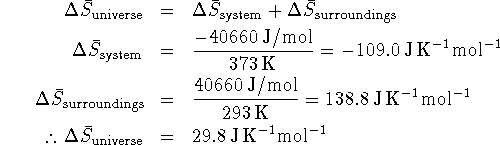
Since the change in entropy of the universe is positive for this process, it is spontaneous.
- First, use the density to determine the mass of ethanol
produced per hour:
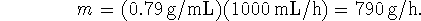
The water is warmed by the condensation which releases
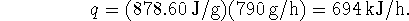
- Again, we need to calculate the mass of water from the
volume which passes through the condenser in an hour:
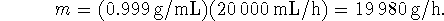
Now calculate the temperature rise:
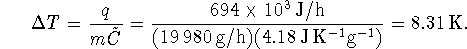
The final temperature is therefore
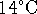 .
.