Because the expansion is reversible, ![]() . We therefore
want
. We therefore
want
![]()
To do the integral, we need to write P in terms of ![]() :
:
![]()
The integral is therefore
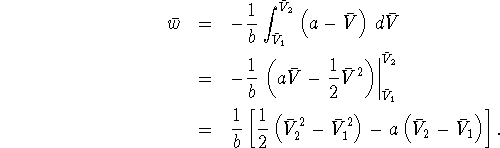
Note that we don't know ![]() and
and ![]() . Instead, we know
. Instead, we know ![]() and
and
![]() , but since we are given the relationship between
, but since we are given the relationship between ![]() and
P, we can calculate the initial and final molar volumes:
and
P, we can calculate the initial and final molar volumes:
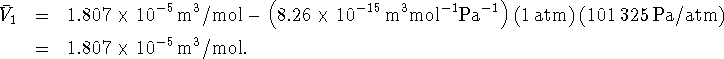
Proceding similarly, we get ![]() . We now have everything we need to calculate
the value of
. We now have everything we need to calculate
the value of ![]() :
:
![]()
This is a tiny amount of energy.
This calculation justifies ignoring the work done on or by solids and
liquids when the pressure is changed by reasonable amounts.