Next: Useful formulas
Up: Back to the old Chemistry 2720 index
Chemistry 2720, Fall 1996, Test 2
All questions are equally weighted.
Answer all questions in this part.
- Calculate the vapour pressure of sodium metal at
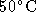 .
. - 0.8246g of a newly isolated protein is dissolved in 100.00mL of
water. The solution has a density of 1.034g/mL and
rises 1.05cm in a capillary tube osmometer at 298K. What
is the molar mass of the protein?
- Suppose that a glucose transporter in a cell is powered by ATP
hydrolysis at 310K:

Two glucose molecules are transported into the cell for every
ATP hydrolyzed. The ratio of ATP to ADP in the cell is
15 and the concentration of phosphate is 5mmol/L. (Both are held
constant by homeostatic mechanisms.) If the extracellular
glucose concentration is 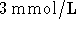 , what is the
maximum glucose concentration which can be obtained in the cell?
, what is the
maximum glucose concentration which can be obtained in the cell?
-
The solubility product of lead (II) iodide is
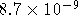 at 298K.
Use Debye-Hückel theory to calculate the solubility of this
compound in water.
at 298K.
Use Debye-Hückel theory to calculate the solubility of this
compound in water.
Answer one of the following questions.
- Derive the differential of G from first principles.
Use your differential to relate the derivatives to G to
thermodynamic variables.
- Very few reactions are exactly thermoneutral. Suppose however
that you are studying a reaction in which the equilibrium
constant only increases by one part in 100000 (i.e.
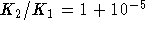 ) when the
temperature is raised from
) when the
temperature is raised from 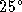 to
to 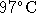 .
Write
.
Write 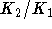 as 1+x and use Taylor's theorem to find an
approximation to
as 1+x and use Taylor's theorem to find an
approximation to 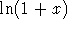 valid for small x. Use this
result to calculate
valid for small x. Use this
result to calculate 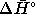 for the reaction.
for the reaction. - Suppose that you want to make compound C by the
gas-phase reaction

but the above reaction produces a very poor yield under normal
conditions. How might you try to improve the yield of C?
Describe at least two different methods.
Next: Useful formulas
Up: Back to the old Chemistry 2720 index
Marc Roussel
Thu Nov 21 12:07:09 MST 1996
![]()
![]() , what is the
maximum glucose concentration which can be obtained in the cell?
, what is the
maximum glucose concentration which can be obtained in the cell?