
Up: Back to the old Chemistry 2720 index
Chemistry 2720 Practice Problems on Statistical Thermodynamics
- Suppose that a system has a ground state with degeneracy
W and that there are no accessible
higher-energy states. Show that the
entropy of this system is just
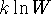 . (This is the Boltzmann
formula for the entropy. In addition to the simple system
described here, the Boltzmann formula gives the entropy of a
system of known total energy and volume. In that case, W is
the number of different ways of rearranging the system without
changing the energy or volume.)
. (This is the Boltzmann
formula for the entropy. In addition to the simple system
described here, the Boltzmann formula gives the entropy of a
system of known total energy and volume. In that case, W is
the number of different ways of rearranging the system without
changing the energy or volume.) -
- Consider a two-level system in which neither energy level is
degenerate. Let the upper level have an energy
 relative to
the lower level. At what temperature does the population in the
upper level become significant relative to that of the lower
level?
relative to
the lower level. At what temperature does the population in the
upper level become significant relative to that of the lower
level? - Work out the internal energy and entropy of this system.
Marc Roussel
Mon Dec 2 14:43:55 MST 1996
 relative to
the lower level. At what temperature does the population in the
upper level become significant relative to that of the lower
level?
relative to
the lower level. At what temperature does the population in the
upper level become significant relative to that of the lower
level?