Next: Thermodynamic data at 298K
Up: Back to the old Chemistry 2720 index
Chemistry 2720, Fall 96, Assignment 2
Due: November 5
All questions are equally weighted. Note that you can use any
equations presented in class or in the textbook without rederiving it.
- What is the pH of a 2mol/L solution of acetic acid in water
at 298K?
- In active transport, a reaction with a large negative free
energy change is used to drive a nonspontaneous transport
process. The reverse is also possible: Spontaneous flow
down a concentration gradient
can be used to drive nonspontaneous chemical reactions. This is
called flow-coupled synthesis. For
example, suppose that a cell somehow creates and maintains
a proton gradient
such that
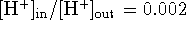 . (Such a gradient can only be maintained by active
transport. Wheels within wheels within wheels...)
This proton gradient is used to drive the synthesis
of 3-phosphoglycerate (PG):
. (Such a gradient can only be maintained by active
transport. Wheels within wheels within wheels...)
This proton gradient is used to drive the synthesis
of 3-phosphoglycerate (PG):
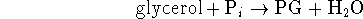
For this reaction,
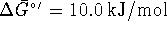 at 298K.
What is the maximum ratio of PG to glycerol which can be
maintained in a single-cell organism living at
at 298K.
What is the maximum ratio of PG to glycerol which can be
maintained in a single-cell organism living at
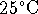 by coupling the spontaneous inward flow of protons
to the synthesis of PG if the phosphate activity in the cell
is
by coupling the spontaneous inward flow of protons
to the synthesis of PG if the phosphate activity in the cell
is 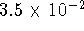 ?
?
- What is the melting point of tin in a vacuum? At 1atm,
tin melts at
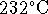 . The densities of solid
and liquid tin are, respectively, 7.18 and 6.99kg/L. The enthalpy
of melting is 60.7kJ/kg at the normal melting point. Hint:
A crucial piece of data can be derived from the
equilibrium condition.
. The densities of solid
and liquid tin are, respectively, 7.18 and 6.99kg/L. The enthalpy
of melting is 60.7kJ/kg at the normal melting point. Hint:
A crucial piece of data can be derived from the
equilibrium condition. - The enthalpy of vaporization of toluene at
its normal boiling point of
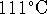 (at 1atm)
is 33kJ/mol. Calculate the boiling point of toluene in
Lethbridge where the atmospheric
pressure is generally about 0.89atm.
(at 1atm)
is 33kJ/mol. Calculate the boiling point of toluene in
Lethbridge where the atmospheric
pressure is generally about 0.89atm. - Relate the formula
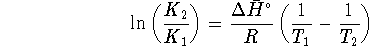
to Le Chatelier's principle. Consider both endothermic and
exothermic reactions.
Marc Roussel
Sun Oct 27 17:12:01 MST 1996
![]()
![]() at 298K.
What is the maximum ratio of PG to glycerol which can be
maintained in a single-cell organism living at
at 298K.
What is the maximum ratio of PG to glycerol which can be
maintained in a single-cell organism living at
![]() by coupling the spontaneous inward flow of protons
to the synthesis of PG if the phosphate activity in the cell
is
by coupling the spontaneous inward flow of protons
to the synthesis of PG if the phosphate activity in the cell
is ![]() ?
?![]()