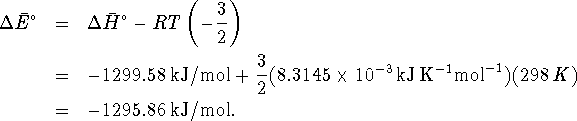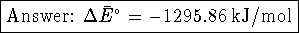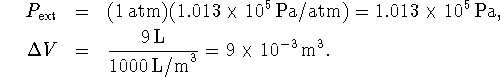
![]()
![]()
The heat required to bring the water to its boiling point is therefore
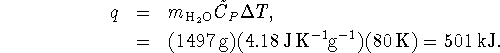
This heat is supplied by a 1500W heating element. A Watt is a Joule per second so
![]()
![]()
- You have to solidify the molten lead. This requires the
removal of
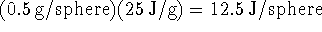 of heat, i.e. the water gains
this much heat.
of heat, i.e. the water gains
this much heat. - The spheres cool from
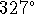 C to
C to 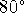 C.
Relative to the lead spheres, this process yields
C.
Relative to the lead spheres, this process yields
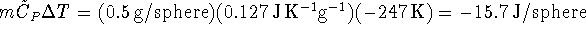 of heat. Thus the water gains
of heat. Thus the water gains 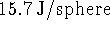 .
.
![]()
Therefore
![]()
![]()
![]()
Note that this calculation assumes 100% efficiency of all the processes involved (muscle work, extraction of nutrients from food, etc.). Nevertheless, it's safe to conclude that it won't be necessary to eat double portions.
![]()
- First, balance the reaction:
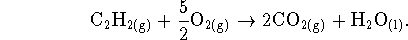
For this reaction,
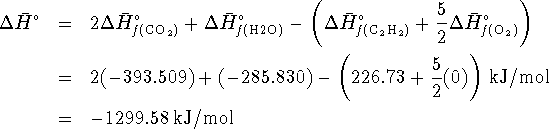
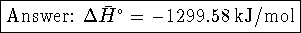
- By definition,
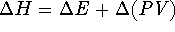 .
Therefore
.
Therefore 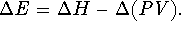 For ideal gases, PV=nRT while for solids and liquids,
the product PV is insignificantly small. Accordingly,
For ideal gases, PV=nRT while for solids and liquids,
the product PV is insignificantly small. Accordingly,
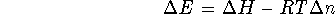
where
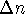 is the change in the number of moles of
gas during the reaction. For this reaction (as written),
there are
is the change in the number of moles of
gas during the reaction. For this reaction (as written),
there are
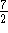 reactant molecules and 2 product molecules
so
reactant molecules and 2 product molecules
so 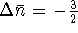 . That gives
. That gives