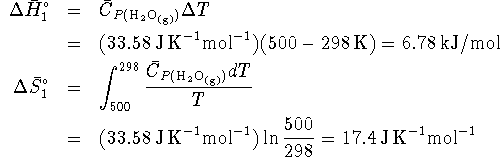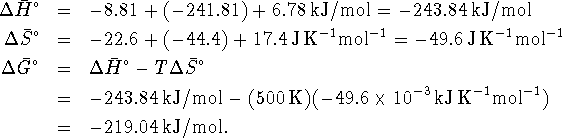- 2% of the maple sap is sugar so we want

The mass of sap required is therefore 5kg.
- Sap is mostly water. We need to raise the temperature
of the water from
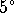 to
to 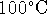 and
then boil it away. Note that there is 4.9kg of water
(98% of 5kg)
but, guessing that the heat capacity of sugar is similar
to that of water, we could reasonably use 5kg as the
mass of water to heat.
and
then boil it away. Note that there is 4.9kg of water
(98% of 5kg)
but, guessing that the heat capacity of sugar is similar
to that of water, we could reasonably use 5kg as the
mass of water to heat.
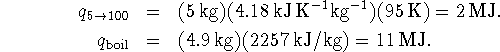
The total heat which must be provided is therefore
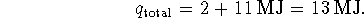
This heat comes from burning decane so
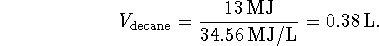
- Lavoisier's proposal was essentially that cells are
heat engines. The maximum efficiency of a heat engine operating
between
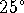 and
and 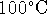 is
is
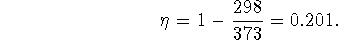
The heat to run this engine is provided by burning glucose. The heat of combustion of glucose is just
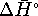 for the reaction
for the reaction
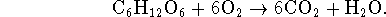
Looking at the data sheet, we see that we have limited information at our disposal. We don't have the heat capacity of glucose so we can't work out the enthalpy of combustion at
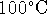 , which is what we
really need. (The fire would be burning in the hot
organelle in this model.) We also only have data
gaseous oxygen and carbon dioxide whereas the reactions in
a cell would certainly involve the dissolved forms.
Our numbers are therefore going to give only a rough
approximation, but of course the maximal efficiency
calculated above can only give us wildly optimistic
estimates anyway. (Obviously, I don't expect you to tell
me all this on an exam.)
, which is what we
really need. (The fire would be burning in the hot
organelle in this model.) We also only have data
gaseous oxygen and carbon dioxide whereas the reactions in
a cell would certainly involve the dissolved forms.
Our numbers are therefore going to give only a rough
approximation, but of course the maximal efficiency
calculated above can only give us wildly optimistic
estimates anyway. (Obviously, I don't expect you to tell
me all this on an exam.)
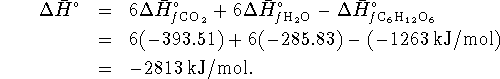
The efficiency is the work performed divided by the heat input. Therefore
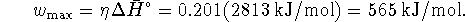
- Isothermal free energy machines are far more efficient
than heat engines.
The maximum work for an isothermal free energy machine
is calculated by computing the change in free energy:
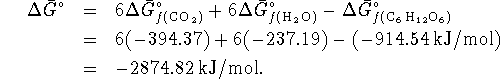
The maximum work is therefore
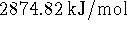 , or over five times as much
as is available from the equivalent heat engine.
, or over five times as much
as is available from the equivalent heat engine.
![]()
where G represents a glucose molecule.
![]() for this process is -31.3kJ/mol since the
standard free energy of formation of glucose is the same inside
as outside the cell. The maximum ratio of glucose in to glucose
out is maintained when
for this process is -31.3kJ/mol since the
standard free energy of formation of glucose is the same inside
as outside the cell. The maximum ratio of glucose in to glucose
out is maintained when ![]() . (This means that all
of the free energy from the reaction is used to transport
glucose.)
Therefore
. (This means that all
of the free energy from the reaction is used to transport
glucose.)
Therefore
![]()
assuming that the activity of water is 1 and with the understanding that each concentration term should be divided by 1mol/L to convert it to an activity. Therefore
![]()
so that
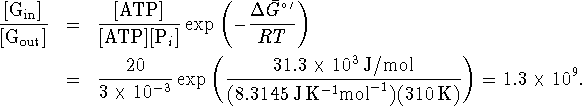
- The vapour pressure is related to the evaporation
process:

The equilibrium constant for this process is

so the equilibrium constant is the vapour pressure. The standard change in free energy is
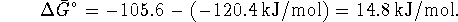
Therefore
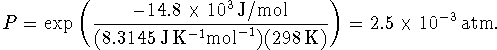
(I have cheated slightly and converted from the dimensionless activity to pressure directly.)
- There are at least two ways to solve this problem:
- Method 1: At the temperature at which a
phase change occurs,
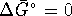 .
This implies that
.
This implies that 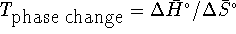 .
For this process
.
For this process
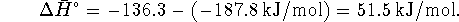
We have
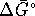 at 298K and
at 298K and
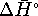 so
so
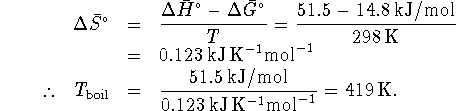
If you prefer the Celcius scale, that's about
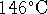 .
.
- Method 2: Since the vapour pressure is just an
equilibrium constant, we can use the equation
which relates equilibrium constants to
temperature. At the boiling point, the vapour
pressure is 1atm.
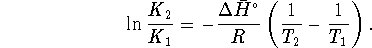
In our case, let the label
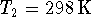 .
Calculate
.
Calculate 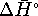 as above and
solve for
as above and
solve for 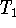 :
:
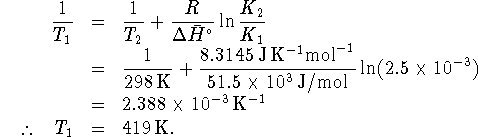
- Method 1: At the temperature at which a
phase change occurs,
![]()
Since both enthalpy and free energy are state functions, we imagine converting hydrogen and oxygen at 500K to steam at 500K by the following path:
- Cool the hydrogen and oxygen from 500K to 298K.
For this step,
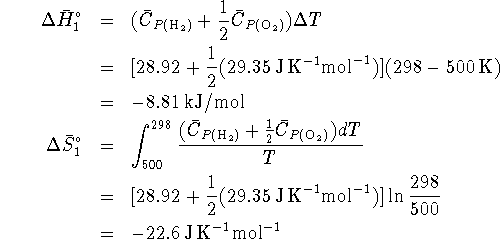
- React the hydrogen and oxygen to form steam at 298K:
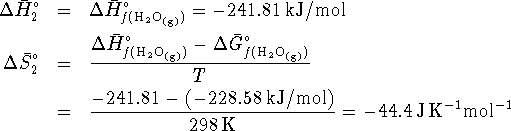
- Heat the steam from 298K to 500K.