- How much sap does it take to make 100g of maple sugar?
- If the sap is heated by burning decane (enthalpy of
combustion: -34.56MJ/L),
how much fuel must be burned to make 100g of maple sugar
from sap at
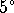 C (a typical harvest temperature)?
C (a typical harvest temperature)?
- Very few cells can survive temperatures in excess of
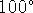 C.
Suppose that
cells converted heat to work directly in some organelles
which maintained themselves at this temperature, but
were surrounded by cytoplasm at
C.
Suppose that
cells converted heat to work directly in some organelles
which maintained themselves at this temperature, but
were surrounded by cytoplasm at 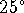 C. What is
the maximum amount of work which could be obtained in
this manner per mole of glucose burned? (Assume that
the combustion produces liquid water rather than steam
as its end product.)
C. What is
the maximum amount of work which could be obtained in
this manner per mole of glucose burned? (Assume that
the combustion produces liquid water rather than steam
as its end product.) - Why is this not a very smart way for cells to produce work? What is the maximum work which can be obtained under standard conditions from the oxidation of glucose using a better strategy?
![]()
( ![]() kJ/mol at 310K)
how large a ratio of glucose concentrations can the cell maintain
across its outer membrane?
Take the ratio of ATP to ADP in the cell to be 20
and the activity of phosphate to be
kJ/mol at 310K)
how large a ratio of glucose concentrations can the cell maintain
across its outer membrane?
Take the ratio of ATP to ADP in the cell to be 20
and the activity of phosphate to be ![]() .
Assume that one glucose molecule is transported for every ATP
hydrolyzed.
.
Assume that one glucose molecule is transported for every ATP
hydrolyzed.
- What is the vapour pressure of hydrogen peroxide at 298K?
- Predict the normal boiling point of hydrogen peroxide.