
Up: Back to the old Chemistry 2720 index
Chemistry 2720, Fall 1995 Final Examination
Answer all questions in this section, with an aggregate value of 27
marks.
- Balance the following redox reactions:
- Permanganate (MnO
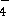 ) reacts
with sulphur dioxide (SO
) reacts
with sulphur dioxide (SO 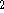 ) to form manganese(II) ions
and HSO
) to form manganese(II) ions
and HSO 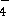 in acidic solution.
in acidic solution. - Solid bismuth(III) hydroxide
(Bi(OH)
 ) reacts with the
SnO
) reacts with the
SnO 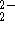 anion to form SnO
anion to form SnO 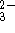 and metallic
bismuth in basic solution.
and metallic
bismuth in basic solution.
- The solubility
of gases in water generally decreases with increasing temperature.
Explain this observation using thermodynamics.
- A fuel cell operates on liquid ethanol at 298K. Oxygen is supplied
at a constant pressure of 0.21atm and carbon dioxide is
removed at such a rate that its pressure is an essentially
constant 0.1atm.
- What voltage does this fuel cell generate?
- If the cell
is improperly operated, it is also possible
for it to generate carbon monoxide instead of carbon
dioxide. Assuming that the carbon monoxide pressure is
0.1atm and that the cell otherwise operates under
identical conditions, what is the operating voltage?
- Mercury is used to make dental filling amalgam. When it is first made,
dental amalgam is essentially a solution
of silver and tin in liquid
mercury.
- Assume
that the solution contains a ratio of Hg:Ag:Sn of
24:15:5 and calculate the equilibrium
vapour pressure of mercury over this solution at
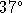 C.
C. - Assume
that the mercury vapour behaves as an ideal gas.
The maximum short-term occupational exposure to mercury
allowed is 0.1mg/m
 . According to your
calculation, are traditional mercury
amalgam dental fillings safe?
The molar mass of mercury is 200.59g/mol.
. According to your
calculation, are traditional mercury
amalgam dental fillings safe?
The molar mass of mercury is 200.59g/mol.
- 3.20mg of a protein of unknown structure
is dissolved in
100g of water. The osmotic pressure of this solution causes
a capillary rise of 1.3mm of the solution when a measurement
is made against pure water at 298K. What is the molar mass of
this protein?
- The solubility product
of calcium fluoride is
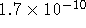 . What is the solubility of calcium fluoride in a 1.5mol/kg
sodium thiocyanate (NaSCN) solution at 298K? Do a full Debye-Hückel
calculation and note that calcium thiocyanate is very soluble.
. What is the solubility of calcium fluoride in a 1.5mol/kg
sodium thiocyanate (NaSCN) solution at 298K? Do a full Debye-Hückel
calculation and note that calcium thiocyanate is very soluble.
Answer any two questions in this section. Do not answer more
than two questions. This section is worth 18 marks.
- How much copper(I) iodide
can be dissolved in a saturated copper(I)
chloride solution at 298K? Do a full Debye-Hückel calculation.
Hint: Calculate the
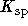 's first.
's first. -
- What is the entropy of vaporization of mercury at
298K?
- What is the boiling temperature of mercury?
- What is the entropy of vaporization of mercury at
its boiling point?
- The electrochemical cell
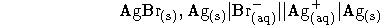
is operated at 298K with a concentration of sodium bromide in
the left half-cell of 0.1mol/kg and a concentration of silver
sulphate in the right half-cell of 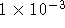 mol/kg.
Reduction occurs in the right half-cell with an
observed output voltage of 0.496V. Considering the effect of
nonideality of the ionic solutions, compute the solubility
product of silver bromide.
Useful fact: The left half-cell reaction is
mol/kg.
Reduction occurs in the right half-cell with an
observed output voltage of 0.496V. Considering the effect of
nonideality of the ionic solutions, compute the solubility
product of silver bromide.
Useful fact: The left half-cell reaction is
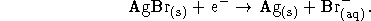
- When 10g of sodium nitrate
(
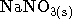 , molar mass 84.99g/mol)
is dissolved in 200g of water, it completely
dissociates and
the temperature of the water drops by
, molar mass 84.99g/mol)
is dissolved in 200g of water, it completely
dissociates and
the temperature of the water drops by 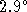 C.
When 10g of nitric acid (HNO, molar
mass 63.01g/mol)
is dissolved in 200g of water, 93% of the acid dissociates to
nitrate and an aqueous proton and the temperature
of the water increases by
C.
When 10g of nitric acid (HNO, molar
mass 63.01g/mol)
is dissolved in 200g of water, 93% of the acid dissociates to
nitrate and an aqueous proton and the temperature
of the water increases by 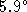 C.
The enthalpy of formation of nitric acid is known from
other experiments to be
-174.1kJ/mol. Assume that the dissociation of the
acid is responsible for most of the temperature rise in
the latter case.
What are the enthalpies of formation of
sodium nitrate and of the nitrate anion?
C.
The enthalpy of formation of nitric acid is known from
other experiments to be
-174.1kJ/mol. Assume that the dissociation of the
acid is responsible for most of the temperature rise in
the latter case.
What are the enthalpies of formation of
sodium nitrate and of the nitrate anion?
R = 8.31441JK 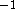 mol
mol 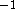 .
.
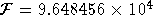 C/mol.
C/mol.
To convert degrees Celcius to Kelvins, add 273.15.
To convert atmospheres to Pascals, multiply by 101325.
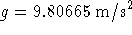
Density of water at 298K: 997.07kg/m 
Heat capacity of liquid water: 4.184Jg  K
K 


Up: Back to the old Chemistry 2720 index
Marc Roussel
Mon Apr 13 15:41:40 MDT 1998
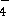 ) reacts
with sulphur dioxide (SO
) reacts
with sulphur dioxide (SO 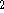 ) to form manganese(II) ions
and HSO
) to form manganese(II) ions
and HSO 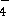 in acidic solution.
in acidic solution. ) reacts with the
SnO
) reacts with the
SnO 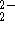 anion to form SnO
anion to form SnO 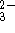 and metallic
bismuth in basic solution.
and metallic
bismuth in basic solution.
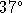 C.
C. . According to your
calculation, are traditional mercury
amalgam dental fillings safe?
The molar mass of mercury is 200.59g/mol.
. According to your
calculation, are traditional mercury
amalgam dental fillings safe?
The molar mass of mercury is 200.59g/mol.

