When NO2 and hydrogen chloride react in the gas phase, the products are HNO3 and NOCl. What is the enthalpy change in this reaction? [5 marks]
- (a)
- Is calcium carbonate stable with respect to calcium oxide
and carbon dioxide at
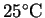 under normal
atmospheric conditions? The pressure of carbon dioxide
in normal air is
under normal
atmospheric conditions? The pressure of carbon dioxide
in normal air is
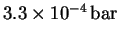 .
[7 marks]
.
[7 marks]
- (b)
- Calcium oxide is an enormously important industrial
chemical used in steel production, in water treatment, and
in the pulp and paper industry, among other
applications.
It has traditionally been produced by heating calcium carbonate
to a high temperature.
Assuming that the partial pressure of carbon dioxide is
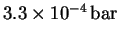 ,
what is the minimum temperature (in degrees Celsius)
at which the
decomposition reaction becomes spontaneous?
[10 marks]
,
what is the minimum temperature (in degrees Celsius)
at which the
decomposition reaction becomes spontaneous?
[10 marks]
Hints: Although the operating temperature may seem rather high, an approximation which we have used for temperatures near standard still gives very good results. You need to simultaneously consider two factors which affect spontaneity to solve this problem.
where Z is the atomic number and