- (a)
- Nuclear physics predicts that there may be a few relatively stable elements with atomic numbers around 114. What would the ground-state electronic configuration of element 114 (ununquadium, Uuq) be? [4 marks]
- (b)
- Predict the ion(s) which might be formed by this element
and give their ground-state electronic configurations.
[4 marks]
Hint: In what group would element 114 be? How does the preceding element in the group behave?
- (c)
- State whether each of the atom and its ion(s) is paramagnetic or diamagnetic. [3 marks]
- (a)
- Show the occupancy of the orbitals in the ground state. [4 marks]
- (b)
- Would you expect Cr2 to be paramagnetic? [1 mark]
- (c)
- The lowest-energy valence orbital has the following
appearance:
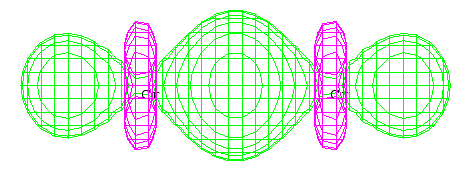
- i.
- Is this a bonding or an antibonding orbital? Explain briefly. [2 marks]
- ii.
- Can you explain the shape of this orbital based on LCAO theory? [2 marks]
- (a)
- N2
- (b)
- CO
- (c)
- NH3
- (d)
- CH4
Bonus: This problem can be solved by doing some straightforward calculations and averaging the results. However, a more accurate value can be obtained by a fitting procedure. One bonus mark may be awarded for successful use of a fitting procedure on these data.