- (a)
- Use a qualitative argument to predict the sign
of the entropy change associated with the combustion
of ethanol at
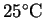 .
[3 marks]
.
[3 marks]
- (b)
- Use the data below to calculate the standard entropy of combustion
of ethanol. [5 marks]
Substance 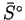 (
(
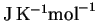 )
)C2H5OH(l) 160.67 CO2(g) 213.74 H2O(l) 69.91 O2(g) 205.138 - (c)
- Suppose that the reaction were carried out at
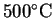 instead of
instead of
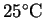 .
Outline the calculations you would have to carry out to
obtain the entropy of combustion at this temperature.
Clearly list all thermodynamic data you would need.
[10 marks]
.
Outline the calculations you would have to carry out to
obtain the entropy of combustion at this temperature.
Clearly list all thermodynamic data you would need.
[10 marks]
Note: Ethanol boils at
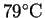 .
.