


Up: Back to the Chemistry 2720 assignments index
Chemistry 2720 Fall 2000 Assignment 2
Due: Tuesday, Sept. 26, 9:25a.m.
- 1.
- Calculate the total enthalpy change for the stoichiometric
conversion of
20g of gaseous carbon
dioxide to carbonic acid (
H2CO3(aq)) by reaction
with liquid water.
[5 marks]
- 2.
- The ideal gas law is only approximately correct. One alternative
commonly used to describe real gases is the virial equation:
If we take
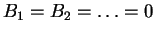 ,
we recover the ideal gas equation. By
taking more and more correction terms, we obtain increasingly
accurate representations of the behavior of a real gas.
,
we recover the ideal gas equation. By
taking more and more correction terms, we obtain increasingly
accurate representations of the behavior of a real gas.
- (a)
- Calculate the work done per mole when an ideal gas expands
isothermally and reversibly
at 273K by a factor of 20. [3 marks]
- (b)
- For carbon dioxide,
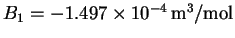 at 273K. Assuming that the
higher-order virial coefficients (B2, B3, etc.)
are all negligibly
small, calculate the work done per mole when carbon
dioxide expands isothermally and reversibly
at 273K from 22 to 440L/mol (a twenty-fold volume
increase).
Would it be appropriate to treat carbon dioxide as an
ideal gas in this situation?
[10 marks]
at 273K. Assuming that the
higher-order virial coefficients (B2, B3, etc.)
are all negligibly
small, calculate the work done per mole when carbon
dioxide expands isothermally and reversibly
at 273K from 22 to 440L/mol (a twenty-fold volume
increase).
Would it be appropriate to treat carbon dioxide as an
ideal gas in this situation?
[10 marks]
- 3.
- A 300g iron block is heated to
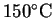 and
then placed in a sealed adiabatic container
with 800g of water
initially at
and
then placed in a sealed adiabatic container
with 800g of water
initially at
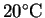 .
What is the final state of
the system? Assume that the pressure is roughly constant at
1atm so that water boils at its normal boiling point. [10 marks]
.
What is the final state of
the system? Assume that the pressure is roughly constant at
1atm so that water boils at its normal boiling point. [10 marks]
- 4.
- Suppose that 25g of sodium hydroxide is dissolved in
200mL of water at
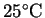 .
What is the
temperature change? According to your calculation, would any special
precautions be necessary in preparing this solution?
[10 marks]
.
What is the
temperature change? According to your calculation, would any special
precautions be necessary in preparing this solution?
[10 marks]



Up: Back to the Chemistry 2720 assignments index
Marc Roussel
2000-09-17
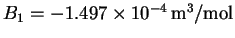 at 273K. Assuming that the
higher-order virial coefficients (B2, B3, etc.)
are all negligibly
small, calculate the work done per mole when carbon
dioxide expands isothermally and reversibly
at 273K from 22 to 440L/mol (a twenty-fold volume
increase).
Would it be appropriate to treat carbon dioxide as an
ideal gas in this situation?
[10 marks]
at 273K. Assuming that the
higher-order virial coefficients (B2, B3, etc.)
are all negligibly
small, calculate the work done per mole when carbon
dioxide expands isothermally and reversibly
at 273K from 22 to 440L/mol (a twenty-fold volume
increase).
Would it be appropriate to treat carbon dioxide as an
ideal gas in this situation?
[10 marks]