


Up: Back to the Chemistry 2710 assignment index
Chemistry 2710 Spring 2001 Assignment 1
Due: Friday, Jan. 12, 9:00a.m.
- 1.
- Evaluate the following derivatives: [2 marks each]
- (a)
-
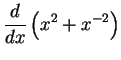
- (b)
-
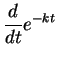
- 2.
- Evaluate the following integrals: [3 marks each]
- (a)
-
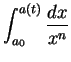 ,
,
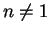
- (b)
-
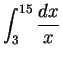 In this case,
simplify your answer as much as possible, but do
not convert it to a floating-point value.
In this case,
simplify your answer as much as possible, but do
not convert it to a floating-point value.
- 3.
- Disulfur dichloride (
S2Cl2)
is used to vulcanize rubber. It is made by
the following reaction:
- (a)
- If 32.0g of sulfur react with 71.0g of chlorine
and assuming the reaction goes to completion,
what mass of disulfur dichloride could be obtained?
[6 marks]
- (b)
- If the chlorine is initially held at a pressure of
90kPa and a temperature of
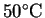 ,
what
volume does it occupy? [3 marks]
,
what
volume does it occupy? [3 marks]
- 4.
- Suppose that HF gas reacts with aluminium metal. The products are
AlF3(s) and hydrogen gas. Calculate the
standard changes in enthalpy and entropy in this reaction.
[6 marks]
| Substance |
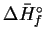 |
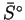 |
| |
(kJ/mol) |
(JK-1mol-1) |
|
Al(s) |
0 |
28.30 |
|
AlF3(s) |
-1510.4 |
66.5 |
|
H2(g) |
0 |
130.680 |
|
HF(g) |
-273.30 |
173.779 |
- 5.
- Find the line of best fit through the following points by
linear regression: [4 marks]
| x |
0.9 |
1.8 |
2.5 |
3.7 |
4.6 |
| y |
3.4 |
5.5 |
7.2 |
10.1 |
12.2 |



Up: Back to the Chemistry 2710 assignment index
Marc Roussel
2001-01-08
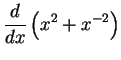
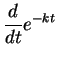
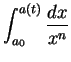 ,
,
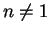
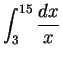 In this case,
simplify your answer as much as possible, but do
not convert it to a floating-point value.
In this case,
simplify your answer as much as possible, but do
not convert it to a floating-point value.
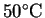 ,
what
volume does it occupy? [3 marks]
,
what
volume does it occupy? [3 marks]