- Let
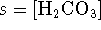 . Then
. Then
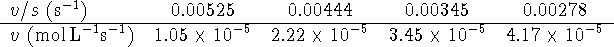
The graph has the following appearance:
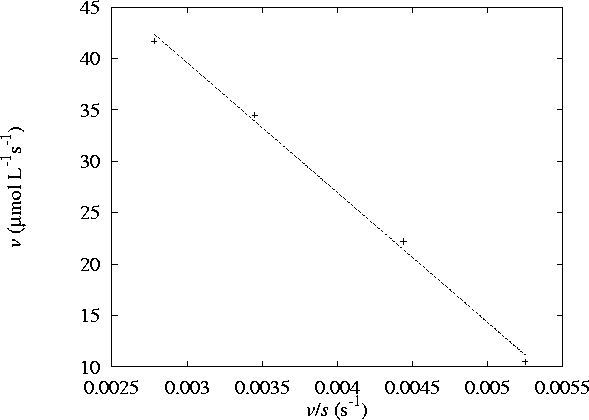 From the slope and intercept, we find
From the slope and intercept, we find 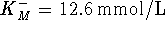 and
and 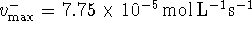 . Since
. Since
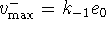 , we have
, we have
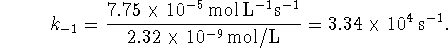
- The Haldane relationship allows us to calculate the
equilibrium constant directly:
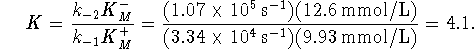
The agreement between the two values isn't spectacular, but then we weren't told much about the conditions under which the enzyme-catalyzed reaction occurred.
-
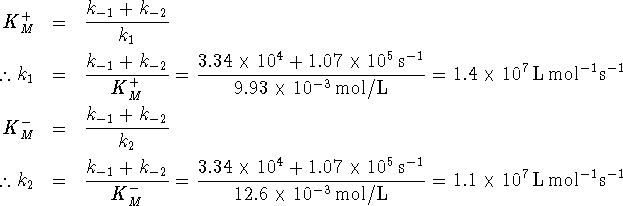
- Oxygen radicals (atoms) can be expected to be quite reactive. Since they appear as intermediates in this reaction, the steady-state approximation should be appropriate.
- One oxygen atom occurs in each of the two reactions, so
we can simply add them. The result is

- As argued above, we can use the steady-state
approximation for O.
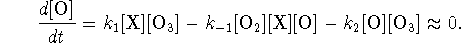
We solve this equation for [O]:
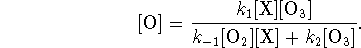
The rate of reaction is
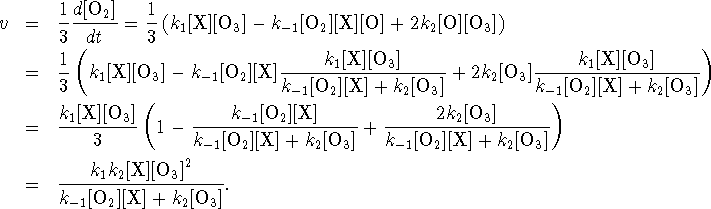
- At high pressure (high [X]),
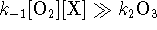 so
that
so
that

The reaction is of the second order with respect to
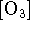 . Additionally, the reaction displays
an order of -1 with respect to
. Additionally, the reaction displays
an order of -1 with respect to 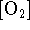 ). In
other words, molecular oxygen inhibits the reaction at
high pressure.
). In
other words, molecular oxygen inhibits the reaction at
high pressure.
On the other hand, at low pressure (low [X]), the rate approaches

and the overall order is 2, with first-order dependencies on both [X] and
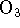 .
Molecular oxygen ceases to exert any significant
influence on the rate of reaction, but the rate becomes
sensitive to the total pressure.
.
Molecular oxygen ceases to exert any significant
influence on the rate of reaction, but the rate becomes
sensitive to the total pressure.
- The
 values are nearly constant, which is
compatible with competitive inhibition. It should also
be the case that a plot of
values are nearly constant, which is
compatible with competitive inhibition. It should also
be the case that a plot of 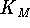 vs
vs 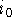 is linear.
is linear.
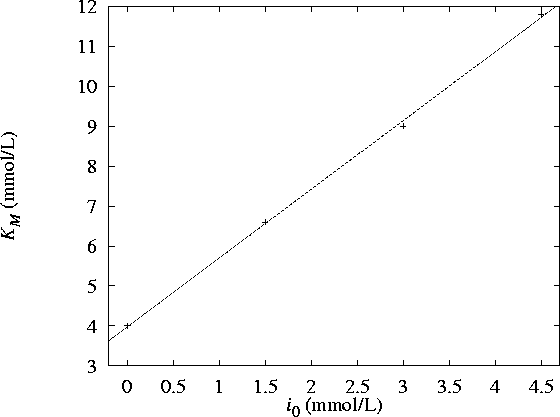 The data do fit a line rather well and so are compatible
with competitive inhibition.
The data do fit a line rather well and so are compatible
with competitive inhibition. -
 is found by averaging the individual
estimates. The result is
is found by averaging the individual
estimates. The result is 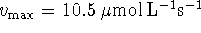 .
.
The plot of
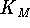 vs
vs 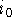 has an intercept
has an intercept 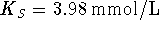 and a slope
and a slope 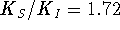 . It follows that
. It follows that 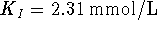 .
.