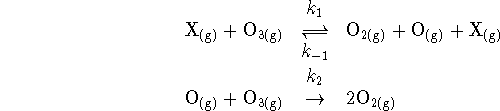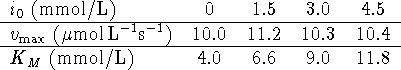Up: Back to the Chemistry 2710 test
index
Chemistry 2710 Spring 2000 Test 2
Name:
Aids allowed: calculator, one  sheet of notes
sheet of notes
Please write your answers in ink. If you have a graphing calculator,
you can use it rather than hand drawing graphs. If you do use your
calculator's graphing capabilities, explain in
detail what you did (what graphs you drew, how you interpreted them,
etc.). If you need to draw graphs by hand, graph paper is included at
the end of this exam. Make sure to label your graphs with
the question number.
- In quiz 8, we calculated the Michaelis constant and
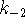 for the reaction
for the reaction
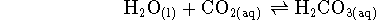
catalyzed by the enzyme carbonic anhydrase. The kinetic
constants of the forward reaction were found to be 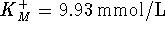 and
and 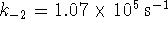 .
Note that water has no
detectable effect on the rate, being present in great excess.
Accordingly, from a kinetic point of view, the reaction is just
.
Note that water has no
detectable effect on the rate, being present in great excess.
Accordingly, from a kinetic point of view, the reaction is just
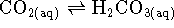 .
.
In an initial rate study of
the reverse reaction catalyzed by a 2.32nmol/L
solution of the enzyme, the following data were obtained:
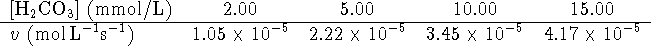
- Calculate
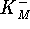 and
and 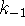 .
[10 marks]
.
[10 marks]
- Calculate the equilibrium constant for the reaction.
From thermodynamics,
we can calculate that
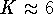 at
at
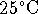 and
and 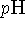 7. Is your
answer in reasonable agreement with this value?
[3 marks]
7. Is your
answer in reasonable agreement with this value?
[3 marks]
- Calculate
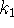 and
and 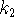 .
[4 marks]
.
[4 marks]
- The decomposition of ozone to form oxygen occurs by the reaction
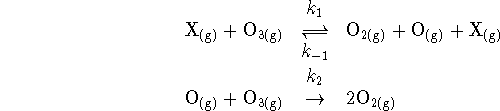
where X can be any of a number of inert species
present in the atmosphere (nitrogen, argon, etc.).
- Identify a particularly reactive species present in this
reaction. Argue, based on the existence of the highly
reactive species identified, that a particular approximation can be
used in deriving the rate law.
[3 marks]
- What is the overall reaction? [2 marks]
- Use an appropriate approximation to derive a rate law for
this reaction. [10 marks]
Note: I expect some degree of simplification in your
answer.
- If you were studying this reaction in controled laboratory
conditions, you could systematically vary the pressure.
How does the order of the reaction with respect to
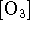 vary with
pressure? Focus particularly on the high- and
low-pressure limits. What other changes in the empirical
rate law
would you observe as a function of pressure? [6 marks]
vary with
pressure? Focus particularly on the high- and
low-pressure limits. What other changes in the empirical
rate law
would you observe as a function of pressure? [6 marks]
- In a series of enzyme inhibition experiments, the following
data were obtained:
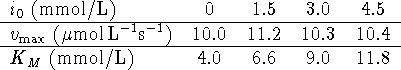
- Are these data compatible with competitive inhibition?
Explain briefly.
[5 marks]
Hint: It might be sensible to use a graphical method to
address at least part of this issue.
- On the assumption that this is an instance of
competitive inhibition, calculate
 ,
,
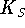 and
and 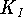 .
[7 marks]
.
[7 marks]

Up: Back to the Chemistry 2710 test
index
Marc Roussel
Mon Mar 27 08:36:02 MST 2000
![]() sheet of notes
sheet of notes
![]()
![]() and
and ![]() .
Note that water has no
detectable effect on the rate, being present in great excess.
Accordingly, from a kinetic point of view, the reaction is just
.
Note that water has no
detectable effect on the rate, being present in great excess.
Accordingly, from a kinetic point of view, the reaction is just
![]() .
.
![]()