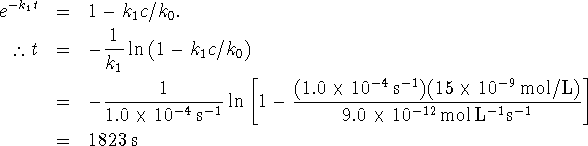- If we look at the first two experiments, we see that the
rate doubles when the ozone concentration doubles.
Comparing the first and third experiments, the rate
triples when the ozone concentration triples. The order
with respect to ozone is therefore one. Similarly, if
we compare the third and fourth experiments or the third
and fifth, we see a simple proportionality between the
rate and nitrogen monoxide concentration. It follows
that the rate law is

The rate constant can be calculated from any one of the experiments, for instance the first one:
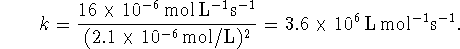
- It could be elementary. It involves only two reactants. One can imagine the transfer of an oxygen atom from one molecule to another occuring in one step. Moreover, the rate law is consistent with this being an elementary reaction.
- B is an intermediate. It should not show up in the
overall reaction. To eliminate it, we add twice the
second reaction to the first. The result is

- With the help of the principle of detailed balance,
we know that at equilibrium, each reaction will be in
equilibrium, i.e. the forward and reverse rates will be
equal. This leads to the equations

If we square the second equation and rearrange, we get

If we now eliminate
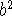 in the first equation using
this one, we obtain
in the first equation using
this one, we obtain

or
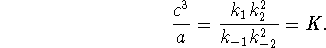
Therefore
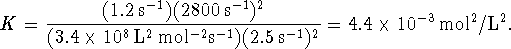
Note that this phenomenological equilibrium constant, unlike the thermodynamic equilibrium constant, has units.
- The integrated rate laws for
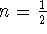 and 1 are
and 1 are
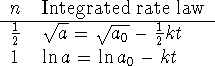
We therefore need to compare plots of
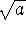 and
and 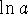 vs t. If one plot is clearly linear while the
others isn't, that will tell us the order of reaction.
First however, we need to calculate a from b.
Since the production of each B requires 2 A's and the reaction
is irreversible, the long-time value of b is half the initial
amount of A, i.e.
vs t. If one plot is clearly linear while the
others isn't, that will tell us the order of reaction.
First however, we need to calculate a from b.
Since the production of each B requires 2 A's and the reaction
is irreversible, the long-time value of b is half the initial
amount of A, i.e. 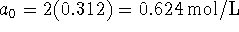 .
Moreover,
.
Moreover, 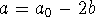 . The data can therefore be transformed
to
. The data can therefore be transformed
to
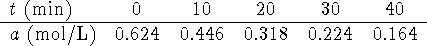
The graphs are as follows:
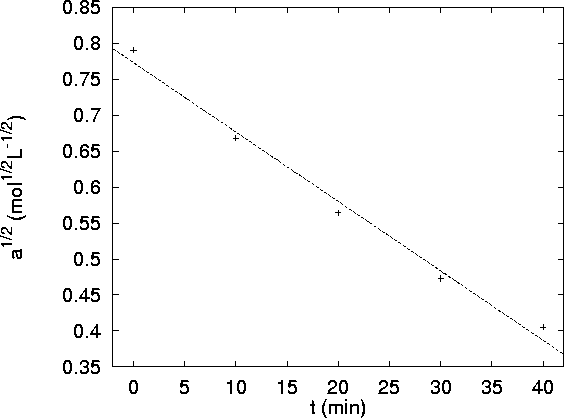
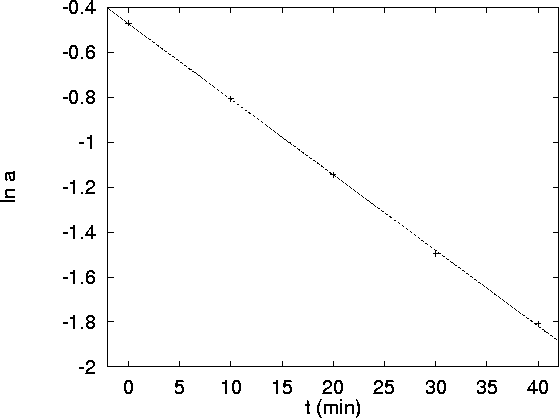
The second plot (
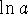 vs t) is clearly linear while
the other isn't. The order of the reaction with
respect to A is therefore one. The rate constant (the
negative of the slope of the plot) is
vs t) is clearly linear while
the other isn't. The order of the reaction with
respect to A is therefore one. The rate constant (the
negative of the slope of the plot) is 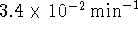 or
or 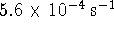 .
. - It's a first-order reaction, but the reaction involves two equivalents of A. This must therefore be a complex reaction with a rate-determining step in which one molecule of A is somehow activated for reaction.
-
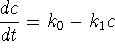
- Clearly,

The time derivative of c(t) is

The right-hand side of the rate equation is
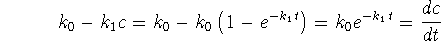
so the differential equation is satisfied.
- Rearrange the solution of the differential equation
to isolate t: