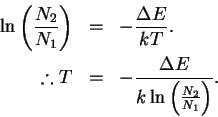Up: Back to the Chemistry 2710 quiz
index
Chemistry 2710 Spring 2000 Quiz 9 Solution
The Boltzmann equation is
We want to solve this equation for T:
We want
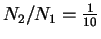 .
The results are (to one significant
figure)
.
The results are (to one significant
figure)
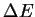 (J)
(J) |
T (K) |
| 10-18 |
30000 |
| 10-20 |
300 |
| 10-22 |
3 |
Marc Roussel
2000-04-07