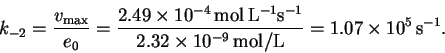| v/s (s-1) | 0.0222 | 0.0200 | 0.0167 | 0.0083 |
| v (
|
|
|
|
|
The best method at your disposal for solving this problem is an
Eadie-Hofstee plot. We first calculate v/s (
s=[CO2]):
The graph has the following appearance:
v/s (s-1)
0.0222
0.0200
0.0167
0.0083
v (
![]() )
)
![]()
![]()
![]()
![]() The data fit the Michaelis-Menten rate law very well. Note however that the
selection of experimental points leaves somewhat to be desired: The
point at
The data fit the Michaelis-Menten rate law very well. Note however that the
selection of experimental points leaves somewhat to be desired: The
point at
![]() is absurdly distant from the
others.
is absurdly distant from the
others.
From the slope
and intercept, we get
![]() and
and
![]() .
Therefore
.
Therefore