


Up: Back to the Chemistry 2710 quiz
index
Chemistry 2710 Spring 2000 Quiz 8
Name:
The enzyme carbonic anhydrase catalyzes both directions of the reaction
A study of the conversion of carbon dioxide to carbonic acid by a
2.32nmol/L solution of carbonic anhydrase yielded the
following initial rate data:
|
[CO2] (mmol/L) |
1.25 |
2.50 |
5.00 |
20.00 |
v (
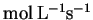 ) ) |
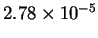 |
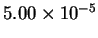 |
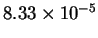 |
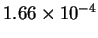 |
Calculate KM and k-2 (the rate constant for the step
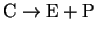 )
for this enzyme.
)
for this enzyme.
Note: Drawing a graph is optional.
Marc Roussel
2000-04-07