Name:
The half-life for the reaction
![]() was studied with equal initial amounts of NO and
was studied with equal initial amounts of NO and ![]() at a
series of different initial pressures (
at a
series of different initial pressures ( ![]() ):
):
![]()
The rate is known to only depend on the concentrations of the two reactants. Find the overall order of the reaction.
Note: Drawing a graph is optional.
For a reaction of general order, 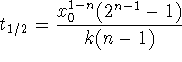 .
.
If equal amounts of NO and ![]() are used, and since they are
used in a 1:1 stoichiometry, the rate law reduces to a simple form. Moreover,
since concentrations and pressures are proportional, they can be used
interchangeably in this kind of problem.
are used, and since they are
used in a 1:1 stoichiometry, the rate law reduces to a simple form. Moreover,
since concentrations and pressures are proportional, they can be used
interchangeably in this kind of problem.