Pressures and concentrations are proportional by
![]()
To completely use 40kPa of A takes 80kPa of B. A is therefore
in excess. When 30kPa of B have been used up, the pressure of A will have
decreased by 15kPa, i.e. the partial pressure of A will have dropped
to ![]() . The rate constant is given
in concentration units. We should therefore convert the initial and final
partial pressures of A to concentration units:
. The rate constant is given
in concentration units. We should therefore convert the initial and final
partial pressures of A to concentration units:
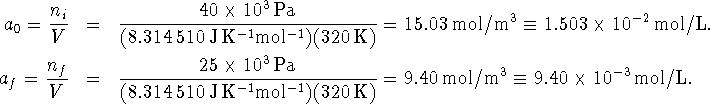
Now we just need to solve the integrated rate law for t:
![]()
The total pressure can be found by stoichiometry. Again we recall that the reaction uses up 15kPa of A (leaving 25kPa) to completely consume the second reactant. When this much A has been used, 15kPa of the product P is formed. Thus the total pressure is
![]()