
Up: Back to the Chemistry 2710 quiz
index
Chemistry 2710 Spring 2000 Quiz 1 solution
The rate of formation of P is the slope of the graph of p as a
function of t. This slope can be found by linear regression. My
calculator gives 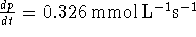 .
The rate of reaction is therefore
.
The rate of reaction is therefore
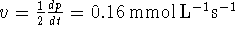 or
or
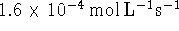 .
.
There are two possible approaches to answering the question of whether
this is truly an initial rate:
- After 10s, only 3.2mmol/L of P has been formed. This
corresponds to using up about 4.8mmol/L of A, less than 1% of
the amount initially present. This is quite a small amount so
the slope probably represents an initial rate.
- If we plot p as a function of t, we get the following graph:
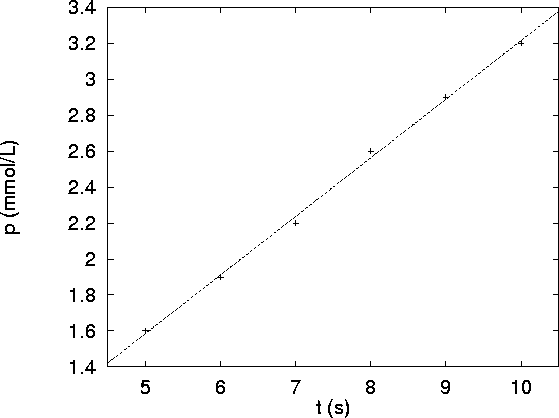
The data fits a line very nicely, so we are still in the initial
rate region.
Marc Roussel
Fri Jan 14 11:08:57 MST 2000
![]() .
The rate of reaction is therefore
.
The rate of reaction is therefore
![]() or
or
![]() .
.
