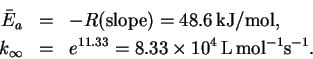Up: Back to the Chemistry 2710 quiz
index
Chemistry 2710 Spring 2000 Quiz 10 Solution
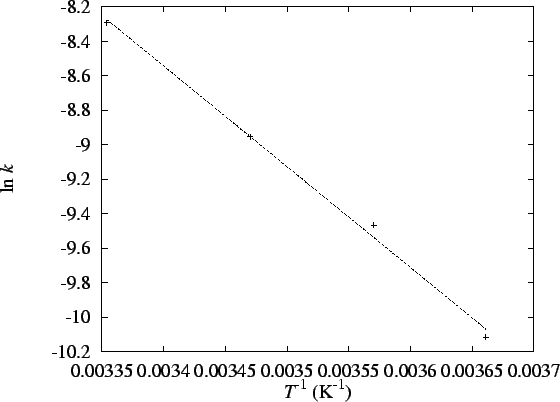
The Arrhenius parameters are obtained by plotting 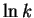 vs T-1.
Our first task is therefore to calculate these quantities:
vs T-1.
Our first task is therefore to calculate these quantities:
| T-1 (
K-1) |
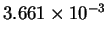 |
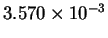 |
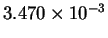 |
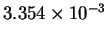 |
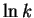 |
-10.117 |
-9.469 |
-8.956 |
-8.294 |
Here is the graph of the data:
The line has slope
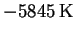 and intercept 11.33. The
activation energy and preexponential factor are therefore
and intercept 11.33. The
activation energy and preexponential factor are therefore
It should be noted that this reaction has unusually small activation
parameters.
Marc Roussel
2000-04-07