-
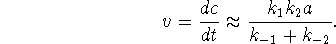
- See course notes.
-
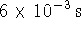
![]()
If the pressure of X is small, the process
![]() might become more significant than activation by the inert gas
so that this step would have to be added to the mechanism.
might become more significant than activation by the inert gas
so that this step would have to be added to the mechanism.
-
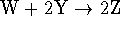
- The answer depends (by a factor of 2) on how you wrote
your overall reaction. If you wrote it as I did, the
rate is
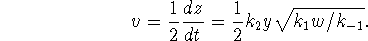
- Most of the time, the SSA gives a rate law of similar
simplicity to the EA, but not in this case. The
SSA value of x satisfies the quadratic equation
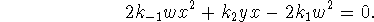
Taking the positive root and computing the reaction velocity as above, we find
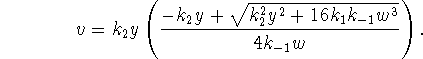
- The bromide ions function as a catalyst.
- Hints: We need to use an equation for conservation of
bromine ions, by analogy to our treatment of enzyme
conservation. We generally need to use a conservation
law when dealing with catalysts.
We also need to write down the overall
reaction to know what we mean by the rate.
The overall reaction is
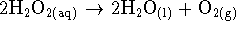 .
The rate is therefore
.
The rate is therefore

where we interpret
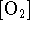 as
as
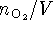 since oxygen is a gas which escapes
from solution. Give or take this minor point, we get
since oxygen is a gas which escapes
from solution. Give or take this minor point, we get
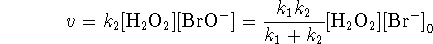
where
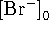 is the total concentration of
bromide in solution.
is the total concentration of
bromide in solution.